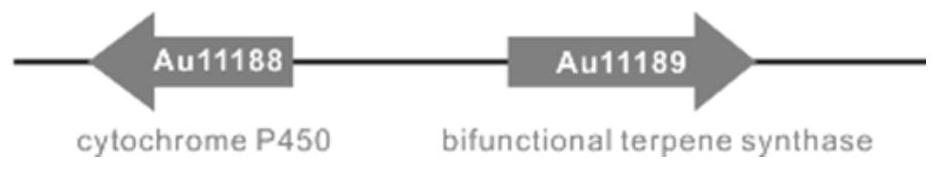
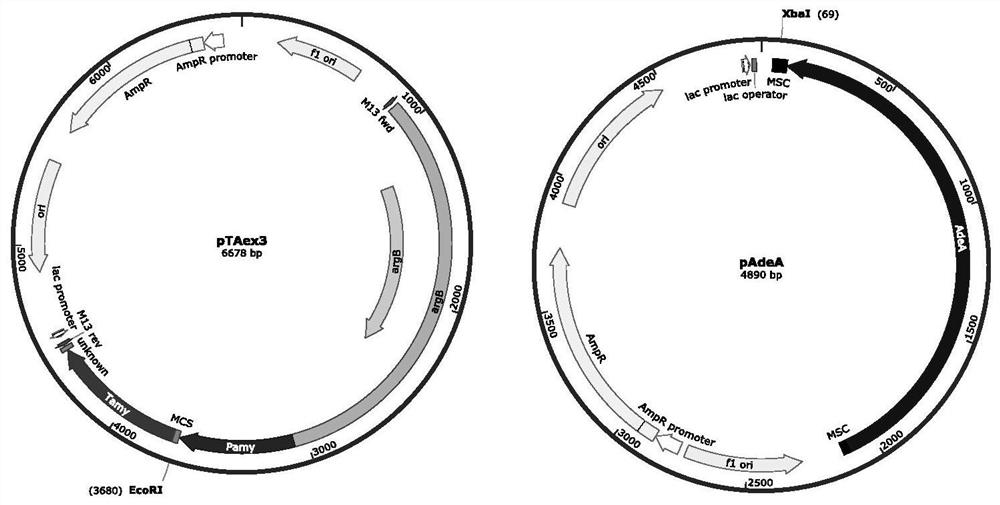
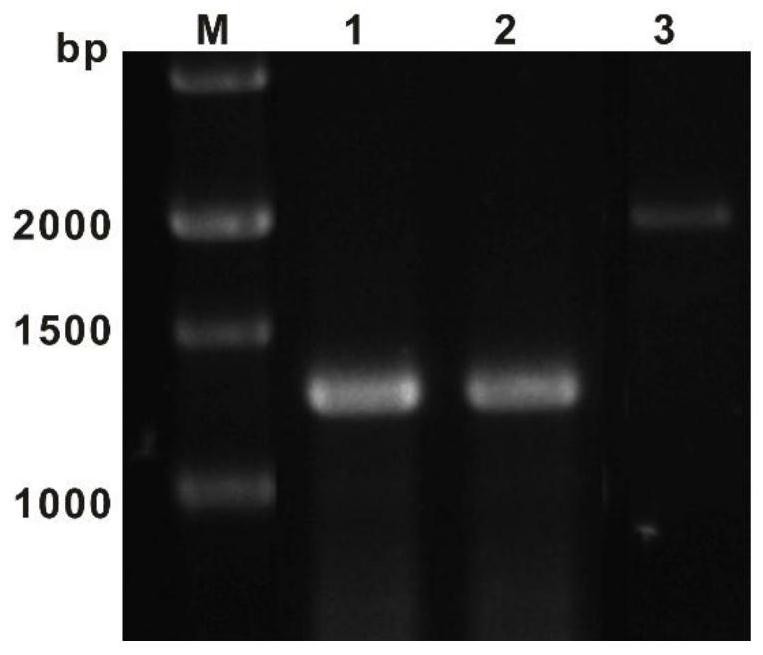
Compound as well as synthetic gene cluster and application thereof
A compound and gene cluster technology applied in the field of microbial medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] [Example 1] Construction of Aspergillus oryzae transformant Ao-AuS1
[0060] Inoculate the spores of the strain 094102 in the fungus II liquid medium, culture at 28°C, 220rpm for 2 days, collect about 20mg of mycelium and add it to a 2mL nucleic acid extraction tube with a small amount of glass beads at the bottom (high temperature sterilization is required to avoid exogenous genes interference). Add 500 μL of sterile water, shake in the nucleic acid extractor for 1 min, then add 500 μL of phenol: chloroform: isoamyl alcohol solution, vortex and mix well, then centrifuge at 12,000 rpm for 10 min, and take the upper aqueous layer as a template for polymerase chain reaction (PCR).
[0061] Referring to the sequence of SAC11189, the primer pair 11189F and 11189R were designed to amplify the gene Au11189. MCLAB 2×High-Fidelity Master Mixon was used to carry out PCR reactions separately, each target gene reaction system was 50 μL, and the components were as follows: 25 μL o...
Embodiment 2
[0064] [Example 2] Aspergillus oryzae transformant Ao-AuS1 fermentation and metabolite detection
[0065] The Aspergillus oryzae transformants obtained in Example 1 were inoculated in 100 mL of DPY liquid medium, cultured at 28° C. and 220 rpm for 7 days, and the mycelium was collected with gauze. Add an equal volume of acetone to the mycelia and soak overnight, ultrasonically crush for 1 hour, repeat three times, and filter through a Buchner funnel to obtain an acetone-water layer. After concentrating under reduced pressure to remove acetone, the aqueous solution was extracted three times with an equal volume of n-hexane; all n-hexane layers were combined and concentrated under reduced pressure to obtain a crude extract. Use 1 mL of n-hexane to redissolve the crude extract and filter for GC-MS detection ( Figure 4-5 ).
[0066] Conclusion: In the gene cluster SAC11189, terpene synthase Au11189 catalyzes the production of four sesquiterpene compounds 1-4 (m / z 340), which ca...
Embodiment 3
[0067] [Example 3] Separation and structure confirmation of precursor compound 1-4
[0068] The Aspergillus oryzae transformant Ao-AuS1 obtained in Example 1 was inoculated in 28L DPY liquid medium, cultured at 28°C and 220rpm for 7 days, the mycelium was collected, and treated according to the method described in Example 2 to obtain a crude extract. Redissolve about 20 g of the obtained crude extract in a small amount of n-hexane, use Qingdao Haiyang 100-200 mesh silica gel to mix the sample, and fill the silica gel column with 200-300 mesh silica gel. Use n-hexane for elution, collect the eluate with glass test tubes, each tube is about 8 mL, and perform GC-MS detection to determine the components. Combine the eluent containing the target sesquiterpene compound, concentrate under reduced pressure and redissolve with 1 mL of acetone, filter and carry out HPLC semi-preparative separation and purification ( Figure 7 ).
[0069] The structures of precursor compounds 1-4 were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com