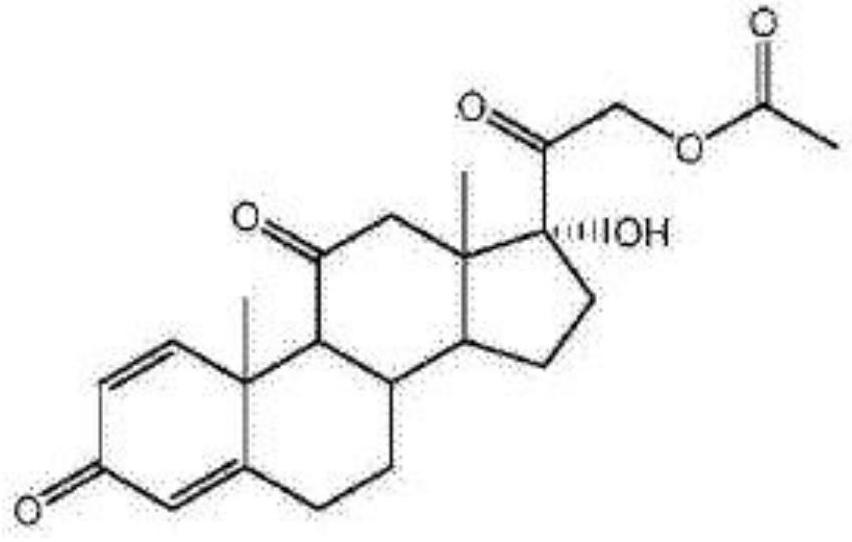
Preparation method of prednisone acetate
A technology of prednisone acetate and seeds, applied in the field of medicine and chemical industry, can solve the problems such as insufficient conversion stability and insufficient stability of substrate dosage, and achieves suitable for large-scale industrial production, high production efficiency and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] The present invention proposes a kind of production fermentation technique of prednisone acetate, comprises the steps:
[0026] (1) Production of the slant of the strain: the composition of the slant medium is 2.0% agar, 1.5% glucose, 2.0% yeast extract powder, pH=6.9-7.6, and the balance is tap water. The culture medium is sterilized by high-pressure steam at 115-125°C for 15-25 minutes, cultured at a constant temperature at 28-33°C for 3-5 days, and stored at 2-8°C until use, and the preservation time does not exceed 45 days.
[0027] (2) Production of 10L of seed medium and 15L of fermentation medium: the components of the seed medium and fermentation medium are glucose 1.2%, corn steep liquor 0.8%, yeast extract powder 0.5%, potassium dihydrogen phosphate 0.2%, pH=6.5 ~7.2, the balance is tap water. The culture medium was sterilized by high-pressure steam at 115-125°C for 15-25 minutes.
[0028] (3) Seed liquid culture: Arthrobacter simplex on the slant of a 250ml...
example 2
[0032] Compared with Example 1, this example is mainly enlarged gradually in scale, and each operation process is the same as that of Example 1, and will not be repeated here. The difference is: by the method in example 1, the Arthrobacter simplex of 2 250ml eggplant bottle slopes is eluted and transferred to 20L seed culture medium (50L fermenter), cultivated for 12.3 hours; wherein 15L seed liquid is transferred to 75L In the fermentation medium (150L fermenter), cultivated for 6.2 hours; add methanol, 9.93kg substrate, soybean oil successively in the same proportion according to the operation of Example 1, adjust parameters, and transform for 32.6 hours; its HPLC conversion rate 96.32%, substrate residue 1.91%, the solid was collected by centrifugation, and 8.23kg of prednisone acetate finished product was obtained through downstream extraction, decolorization, refining, drying and other operations, with a weight yield of 82.88%. The product quality was tested in accordance ...
example 3
[0034] Compared with Example 1, this example is mainly enlarged gradually in scale, and each operation process is the same as that of Example 1, and will not be repeated here. The Arthrobacter simplex of 2 250ml eggplant bottle slopes is eluted by the method in example 1 and transfers in 20L seed medium (50L fermenter), cultivates 11.8 hours; Wherein 15L seed liquid is transferred into 75L fermentation medium (150L Fermentation tank), cultivated for 6.5 hours; add methanol, 10.68kg substrate, soybean oil successively in the same proportion according to the example 1 operation, adjust parameters, convert 34.8 hours, HPLC conversion rate 96.03%, substrate residue 2.05%, centrifuge to collect solid , 8.81 kg of prednisone acetate finished product was obtained through downstream extraction, decolorization, refining, drying and other operations, with a weight yield of 82.49%, and the product quality was tested in accordance with the "Chinese Pharmacopoeia" (2020 edition).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com