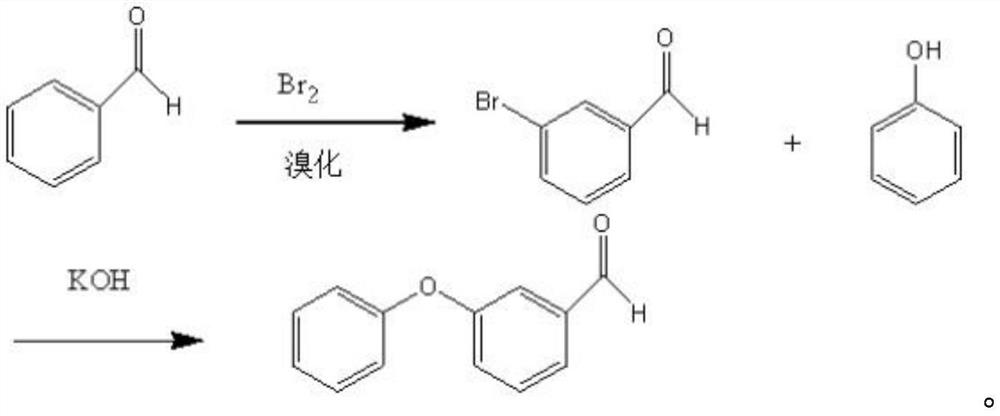
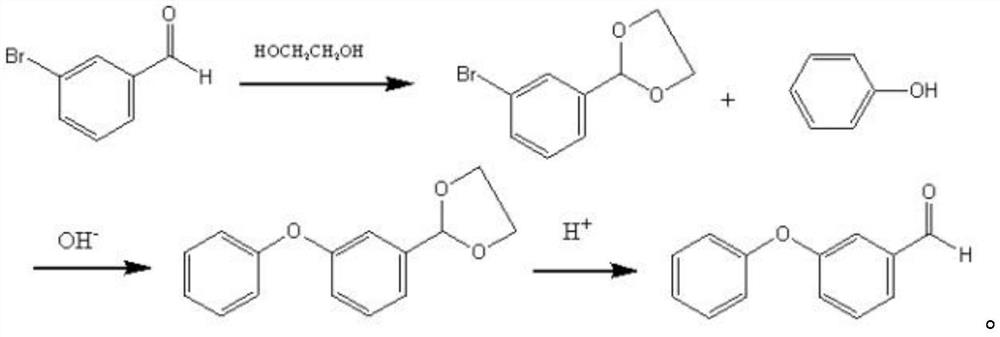
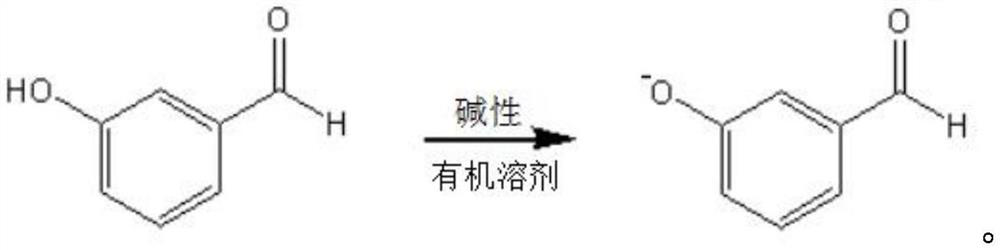
Preparation method of m-phenoxy benzaldehyde
A technology of m-phenoxybenzaldehyde and halobenzene is applied in the field of preparation of m-phenoxybenzaldehyde, can solve problems such as low yield and low purity of m-phenoxybenzaldehyde, and achieves reduction of production cost and simple process The effect of easy operation and cheap and easy to obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a kind of preparation method of m-phenoxybenzaldehyde, comprising the following steps:
[0027] Performing a salt-forming reaction between m-hydroxybenzaldehyde and an alkaline reagent in an anhydrous organic solvent to obtain a salt-forming product reaction solution;
[0028] mixing the salt-forming product reaction liquid with halobenzene, performing a substitution reaction between the salt-forming product and halobenzene under alkaline conditions, and subjecting the obtained substitution reaction liquid to solid-liquid separation to obtain a liquid phase;
[0029] Fractional distillation is performed on the liquid phase, and fractions at 170-190° C. are collected to obtain the m-phenoxybenzaldehyde.
[0030] In the present invention, unless otherwise specified, the components in the preparation method are commercially available products well known to those skilled in the art.
[0031] In the invention, m-hydroxybenzaldehyde and alkaline reagen...
Embodiment 1
[0056] Mix 122.1g (1.0mol) of m-hydroxybenzaldehyde, 244mL of N,N-dimethylformamide and 165.8g (1.2mol) of anhydrous potassium carbonate, and control the pH value of the resulting mixed system to 12. Under heat preservation and stirring for 1h, carry out a salt-forming reaction to obtain a mixture containing an intermediate;
[0057] Under the condition of 90-100°C, 164.8g (1.05mol) of bromobenzene was added dropwise into the mixture at a rate of 2 drops / s, and the intermediate and halobenzene were kept and stirred at 90-100°C for 2 hours to carry out the substitution reaction. After the mixed system was cooled to 30°C, it was filtered, and the obtained filtrate was subjected to fractional distillation, and the fraction at 170-190°C was collected to obtain 160.5 g of m-phenoxybenzaldehyde.
[0058] The product obtained in this example is detected: mass spectrometry MS (ESI), m / z: 199.3 [M+H] + ;
[0059] NMR 1 H NMR (400MHz, CDCl 3 ), δ=7.22(2H, ArH), 6.98(H, ArH), 6.92(2H...
Embodiment 2
[0062] Mix 122.1g (1.0mol) of m-hydroxybenzaldehyde, 244mL of N-methylpyrrolidone and 165.8g (1.2mol) of anhydrous potassium carbonate, control the pH value of the resulting mixed system to 12, and keep stirring at 90-100°C for 1h Perform a salt-forming reaction to obtain a mixture containing intermediates;
[0063] Under the condition of 90-100°C, 164.8g (1.05mol) of bromobenzene was added dropwise into the mixture at a rate of 2 drops / s, and the intermediate and halobenzene were kept and stirred at 90-100°C for 2 hours to carry out the substitution reaction. After the mixed system was cooled to 30°C, it was filtered, and the resulting filtrate was subjected to fractional distillation, and the fraction at 170-190°C was collected to obtain 159.2 g of m-phenoxybenzaldehyde.
[0064] The present embodiment middle phenoxybenzaldehyde yield is 80.4%; The gas phase content GC of m-phenoxybenzaldehyde is 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com