Swine fever antigen epitope peptide and use thereof
An antigen epitope and swine fever technology, applied in the field of biomedicine, can solve the problems of low protein conformation folding accuracy, low antigen epitope abundance, complex purification methods, etc., and achieve excellent immune effect, short cycle time, and simple purification methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 hog fever antigenic epitope peptide
[0043] In this example, four key epitope peptides ER1, ER2, ER3 and ER4 were designed according to the N-terminal 100-550 amino acids of the ASFV P72 antigen.
[0044] ER1 is the amino acid sequence shown in SEQ ID NO.1, which is selected from N-terminal D119-R174 of African swine fever virus protein P72.
[0045] ER2 is the amino acid sequence shown in SEQ ID NO.2, which is selected from N-terminal P240-D305 of African swine fever virus protein P72.
[0046] ER3 is the amino acid sequence shown in SEQ ID NO.3, which is selected from N-terminal G373-P399 of African swine fever virus protein P72.
[0047] ER4 is the amino acid sequence shown in SEQ ID NO.4, which is selected from N-terminal A496-T529 of African swine fever virus protein P72.
[0048] ER1, ER2, ER3 and ER4 can be embedded in the Cap protein of porcine circovirus PCV2, the immunodominant region of hepatitis B core antigen monomer, human ferritin and trim...
Embodiment 2
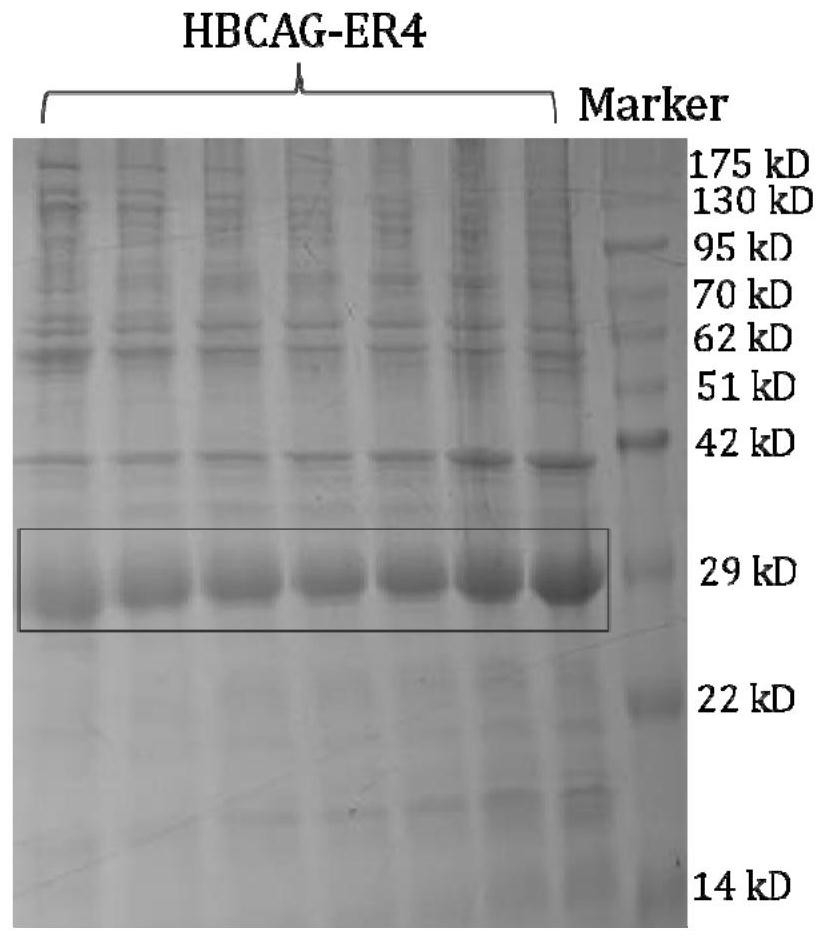
[0049] The preparation of embodiment 2 recombinant antigen HBCAG-ER4
[0050] The preparation of recombinant antigen HBCAG-ER4 comprises the following steps:
[0051] (1) Gene synthesis and molecular construction: embed the gene sequence of the ER4 antigen epitope peptide between 79-80aa of the gene sequence of the HBCAG protein backbone (the amino acid sequence of the HBCAG protein backbone is shown in SEQ ID NO.5), and place The obtained recombinant gene sequence was inserted between NcoI and xhoI of the pET-28a vector to obtain a recombinant plasmid. The synthesis and plasmid construction of the above-mentioned genes were all carried out by Nanjing GenScript Company.
[0052] (2) Plasmid transformation: Add 2 μl of the recombinant plasmid (80ng / μl) described in step (1) to Escherichia coli BL21(DE3) competent cells (purchased from Bomide), and put it on ice for 30 minutes, then 42°C Heat shock for 90 seconds, place on ice again for 2 minutes, add 800 μl of LB medium, plac...
Embodiment 3
[0055] The preparation of embodiment 3 recombinant antigen HBCAG-ER2
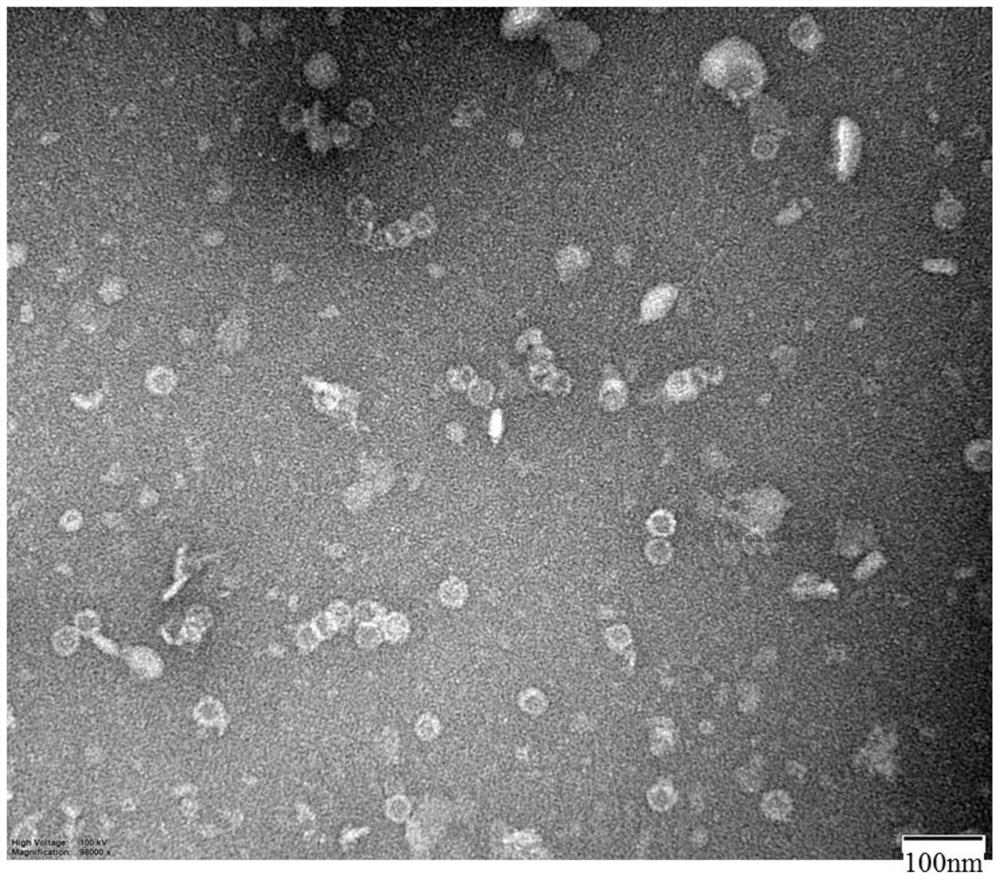
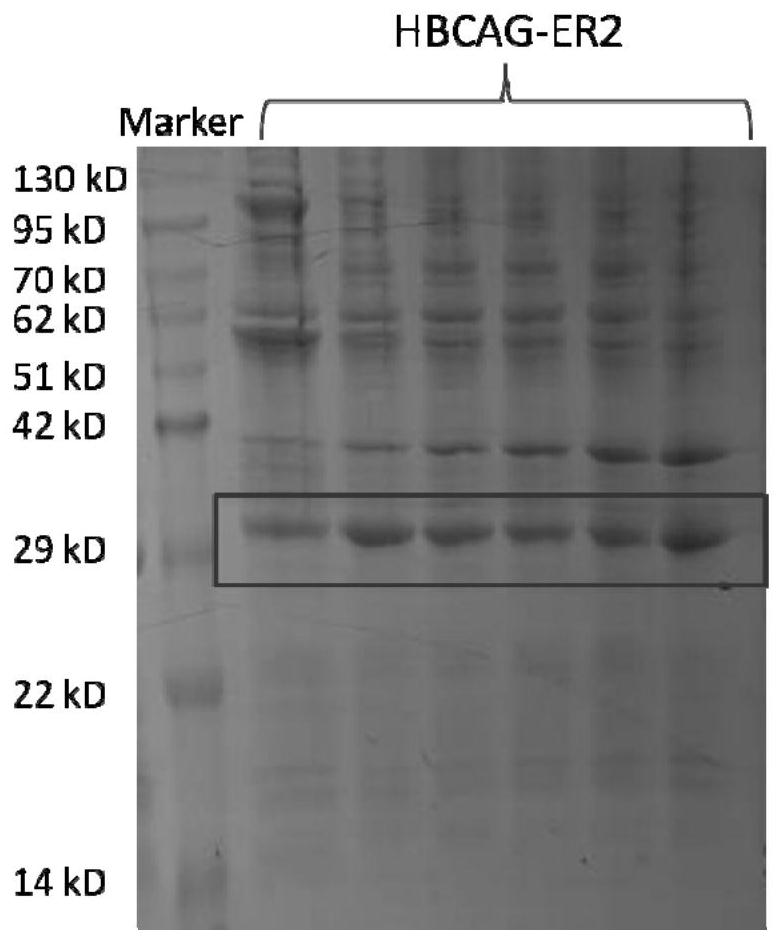
[0056] The preparation method of this embodiment is basically the same as that of Example 2, the only difference is that in step (1) gene synthesis and molecular construction, in order to embed the gene sequence of the ER2 epitope peptide into the HBCAG protein skeleton (the HBCAG protein skeleton is as shown in SEQ The amino acid sequence shown in ID NO.5) is between 79-80aa of the gene sequence, and the obtained recombinant gene sequence is inserted between the pET-28a vector NcoI and xhoI to obtain a recombinant plasmid. The synthesis and plasmid construction of the above-mentioned genes were all carried out by Nanjing GenScript Company. in, image 3 The SDS-PAGE results of the HBCAG-ER2 recombinant antigen are shown, Figure 4 The negative-stained electron microscope picture of the HBCAG-ER2 recombinant antigen is shown. It can be seen that the HBCAG-ER2 recombinant antigen has successfully assembled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com