A kind of Gemini type fluorosurfactant and its preparation method and application
A surfactant and gemini technology, applied in the field of gemini fluorosurfactants and their preparation, can solve problems such as endocrine disruption, neurotoxicity, etc., and achieve the effects of excellent surface activity, high productivity and high solid content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
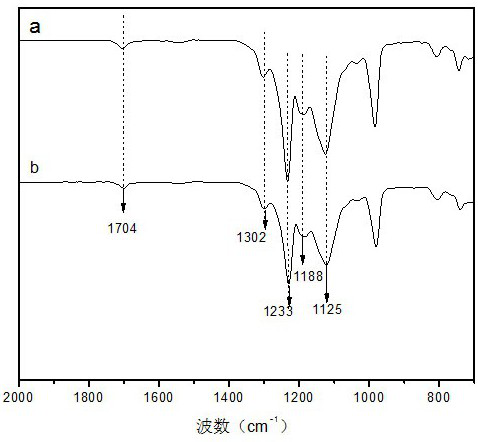
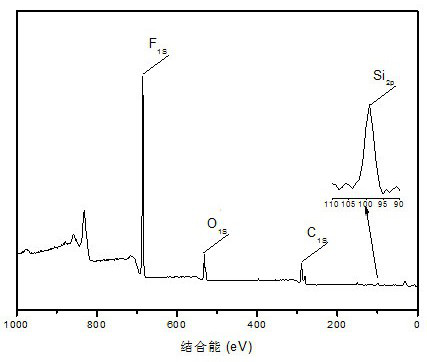
[0063] The present invention further discloses the preparation method of the above-mentioned gemini type fluorine-containing surfactant, first forming a block copolymer by coupling method with perfluoropolyether and chlorinated polyethylene glycol monomethyl ether, and further hydrolyzing the block copolymer Condensation obtains gemini type surfactant, specifically comprises the following steps:
[0064] (1) Alkenylation of perfluoropolyether
[0065] a. Prepare acid-binding agent: by weight, dissolve 4-30 parts of inorganic base in 8-300 parts of water to obtain acid-binding agent;
[0066] b. Substitution reaction: by weight, put into the reactor 50~100 parts of perfluoropolyether alcohol, 0.4~1 part of tetrabutylammonium bromide, 50~3000 parts of fluoroalkylbenzene solvent, 4~50 parts of halogen Substituting allyl group, control the temperature at 20-85°C, add the prepared acid-binding agent dropwise, and the dropping time is 30 minutes to 2 hours; after the addition, keep...
Embodiment 1
[0084] (1) Synthesis of chlorosilanized perfluoropolyether
[0085] 5.6g of potassium hydroxide is dissolved in 20g of deionized water to obtain an acid-binding agent for subsequent use;
[0086] A 500mL three-necked flask was equipped with a thermometer, a reflux condenser and a constant pressure dropping funnel, and 170g of 1,3-bistrifluorotoluene, 80g of number-average perfluoropolyether alcohol (molecular weight: 2000, purchased from HEC Group), and 0.5g of four Butylammonium bromide, 5.3g propylene bromide; after stirring to dissolve completely, raise the temperature to 85°C, add the pre-prepared acid-binding agent dropwise, the dropwise addition time is 30 minutes, and keep warm for 4 hours after the addition;
[0087] After the reaction, stand still and separate layers, discard the upper water layer, add 3g of anhydrous magnesium sulfate to the lower oil layer and dry for 12 hours, filter and remove the desiccant to obtain an alkenylated perfluoropolyether solution, whi...
Embodiment 2
[0100] (1) Synthesis of chlorosilanized perfluoropolyether
[0101] 5.6g of potassium hydroxide is dissolved in 20g of deionized water to obtain an acid-binding agent for subsequent use;
[0102] A 500mL three-necked flask was equipped with a thermometer, a reflux condenser and a constant pressure dropping funnel, and 170g of 1,3-bistrifluorotoluene, 120g of number-average perfluoropolyether alcohol (molecular weight of 3000, purchased from HEC Group), and 0.5g of four Butylammonium bromide, 5.3 g propene bromide. After stirring to dissolve completely, raise the temperature to 85°C, add the pre-prepared acid-binding agent dropwise for 30 minutes, and keep it warm for 4 hours after adding;
[0103] After the reaction, stand still and separate layers, discard the upper water layer, add 3g of anhydrous magnesium sulfate to the lower oil layer and dry for 12 hours, filter and remove the desiccant to obtain an alkenylated perfluoropolyether solution, which is transferred to a 500m...
PUM
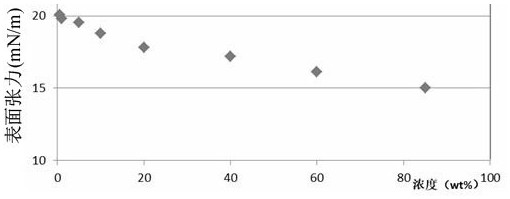
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com