Preparation method of dicyclic sulfite and dicyclic sulfate
A technology of cyclic sulfite and cyclic sulfate, which is applied in the direction of organic chemistry, can solve the problems of high requirements for environmental protection and safety production, long reaction time, and unfriendly environment, so as to improve utilization rate and reduce reaction time. It takes a short time to avoid the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
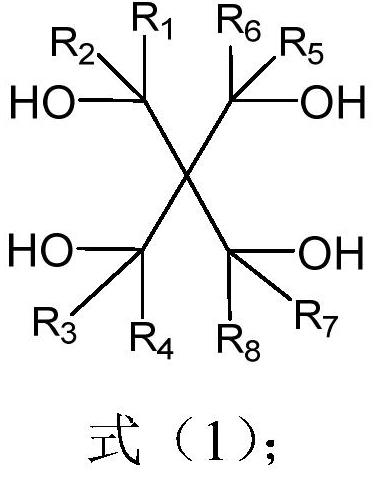
[0041] The invention provides a method for preparing bicyclic sulfite, which is prepared by mixing compound A with thionyl chloride for reaction;
[0042] Compound A has a structure shown in formula (1):
[0043]
[0044] Bicyclic sulfite has a structure shown in formula (2):
[0045]
[0046] Among them, R 1 ~R 8 Each occurrence is independently selected from H, a halogen atom, a cyano group, silane trifluoride, or a hydrocarbon group with 1 to 3 carbon atoms substituted by at least one X;
[0047] X are each independently selected from H, F, silyl, cyano or isocyanate.
[0048] In a concrete example, R 1 ~R 8 Each occurrence is independently selected from H, F, cyano, silane trifluoride, or an alkyl group with 1 to 3 carbon atoms substituted by at least one X.
[0049]In a specific example, compound A is pentaerythritol, 3,3-bis-(1-hydroxy-ethyl)-pentane-2,4-diol, 1,3-difluoro-2,2-dihydroxy Methylpropane-1,3-propanediol, 1,3-difluoro-2,2-bis-(fluoromethylol)prop...
Embodiment 1
[0069] The present embodiment provides a kind of preparation method of bicyclic sulfite and bicyclic sulfate, specifically as follows:
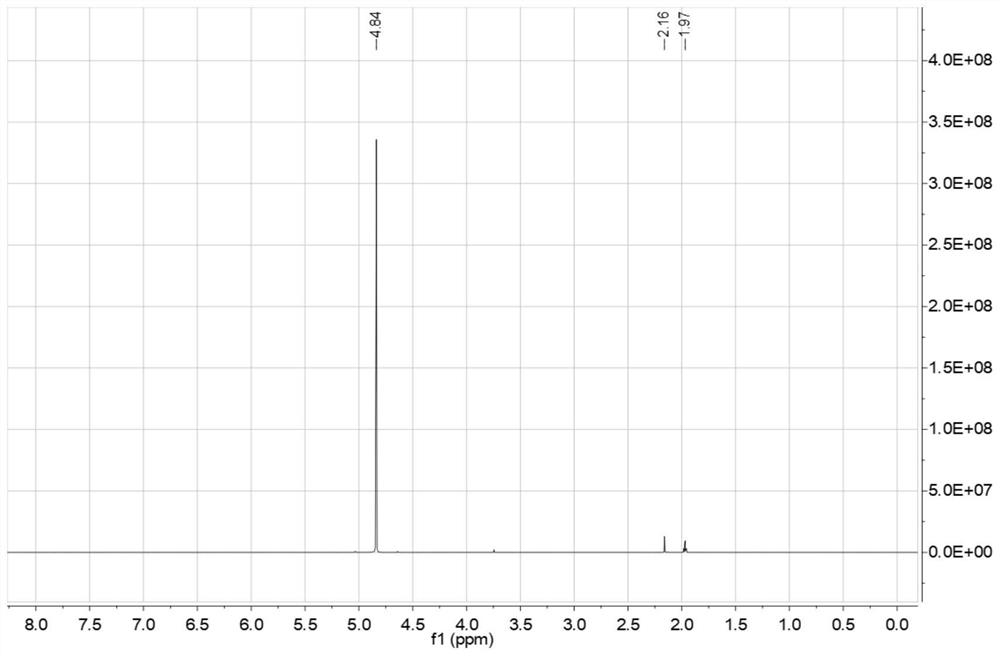
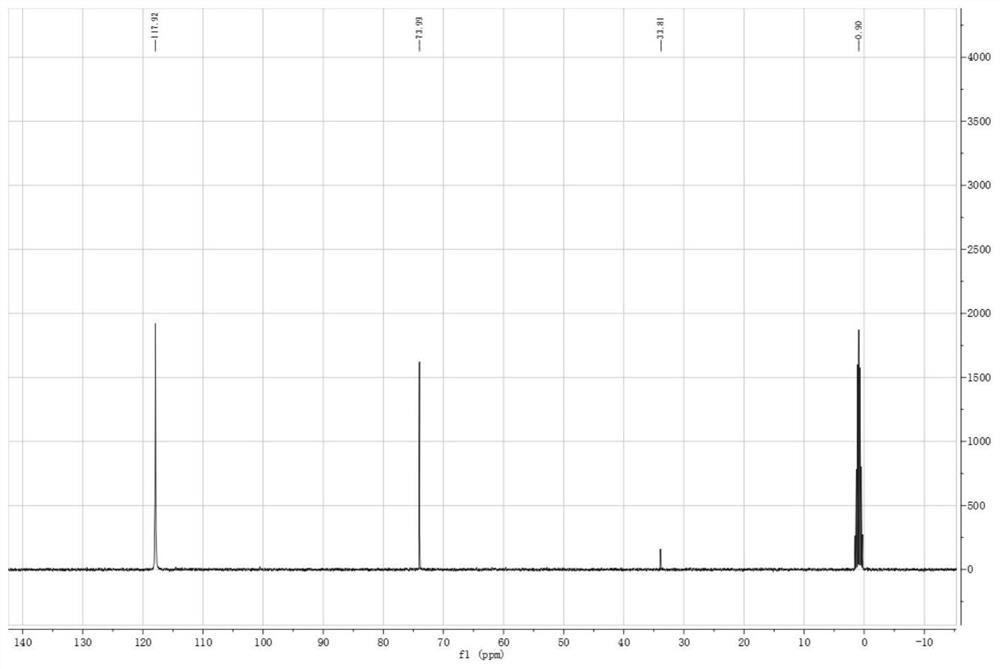
[0070] At room temperature, add thionyl chloride (0.8 mol) and pentaerythritol (0.2 mol) into a reaction flask protected by nitrogen circulation, stir and raise the reaction temperature to 60°C, stir for 6 hours, and cool to room temperature after the reaction is complete. Solid-liquid separation, liquid thionyl chloride recycling, solid bicyclic sulfite (3,9-dioxide-2,4,8,10-tetraoxa-3,9-dithiaspiro[ 5.5] Undecane), the yield is 85.5%, and the purity is 99.23%;
[0071] Subsequently, acetonitrile, bicyclic sulfite (0.171mol), and ruthenium trichloride hydrate (0.3%) were sequentially added to the reaction flask, and after cooling down to -5°C to stabilize, sodium hypochlorite (0.38mol) solution was added dropwise, and the addition was completed After the temperature was maintained at room temperature, after the reaction was completed for 1 ...
Embodiment 2
[0073] The present embodiment provides a kind of preparation method of bicyclic sulfite and bicyclic sulfate, specifically as follows:
[0074] At room temperature, add thionyl chloride (1.0 mol) and pentaerythritol (0.2 mol) into a reaction flask protected by nitrogen circulation, stir and raise the reaction temperature to 40°C, stir for 6 hours, cool to room temperature, and separate the solid and liquid. Liquid thionyl chloride is recycled and used, and the solid is bicyclic sulfite crude product (3,9-dioxide-2,4,8,10-tetraoxa-3,9-dithiaspiro[5.5]dec One alkane), the yield is 86.7%, and the purity is 99.41%.
[0075] Subsequently, dichloromethane, bicyclic sulfite (0.173mol), and ruthenium trichloride hydrate (0.25%) were sequentially added to the reaction flask, and after cooling down to -5°C to stabilize, sodium hypochlorite (0.47mol) solution was added dropwise. After the addition, the temperature was maintained at room temperature for 1 h, the organic phase was collect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com