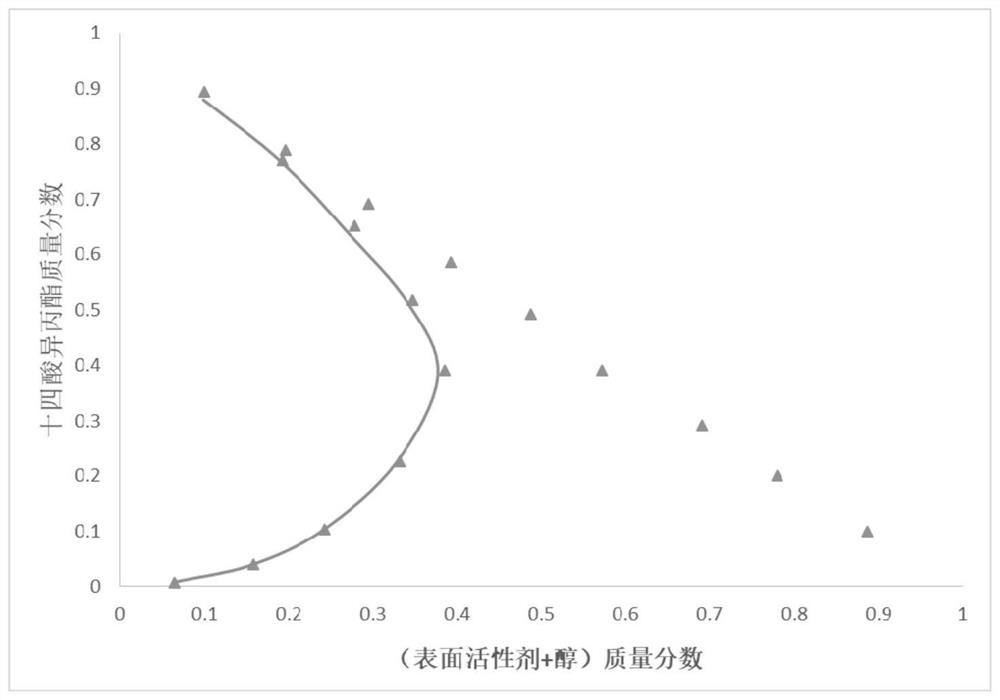
Piperazinyl ionic liquid surfactant as well as synthesis method and application thereof
A technology for surfactants and ionic liquids, applied in the field of piperazine-based ionic liquid surfactants and their synthesis, can solve the problems of industrial application obstacles, high production costs, complicated preparation and purification steps, etc., and achieve easy industrial scale-up and operation Simple, effect of changing thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The synthetic method of piperazine-based ionic liquid surfactant of the present invention, comprises the steps:
[0030] Dissolving piperazine derivatives in an organic solvent, gradually adding long-chain carboxylic acid, reacting at room temperature for 3 to 24 hours, removing the solvent, washing, and drying, to obtain the piperazine-based ionic liquid surfactant; wherein, R 1 is hydroxyethyl, phenyl, hydroxypropyl, formyl, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl or tert-butyl.
[0031] Described long-chain carboxylic acid has following general chemical structure formula: R 2 COOH, where R 2 is the general formula for C n h 2n+1 straight-chain alkyl, n is 9-17.
[0032] The molar ratio of the piperazine derivative to the long-chain carboxylic acid is 1:1.
[0033] The organic solvent is one of methanol, ethanol, ether, methylene chloride, chloroform, carbon tetrachloride, and ethyl acetate.
Embodiment 1
[0035] The synthetic method of the piperazine-based ionic liquid surfactant of the present embodiment is as follows:
[0036] (1) Add 0.1mol 1-ethylpiperazine and 100mL dichloromethane in the round bottom flask;
[0037] (2) After dissolving 0.1mol capric acid in 100mL of dichloromethane, gradually add it to the solution of 1-ethylpiperazine in dichloromethane. During this process, keep the round-bottomed flask in an ice-water bath, then stir at room temperature for 3h, the stirring speed 400rpm;
[0038] (3) Distilled under reduced pressure at room temperature, the obtained solid was washed with ether, filtered, and then vacuum-dried at room temperature for 48 hours to obtain the product 1-ethylpiperazine decanoate with a yield of 96.5%.
[0039] Structural characterization of 1-ethylpiperazine caprate:
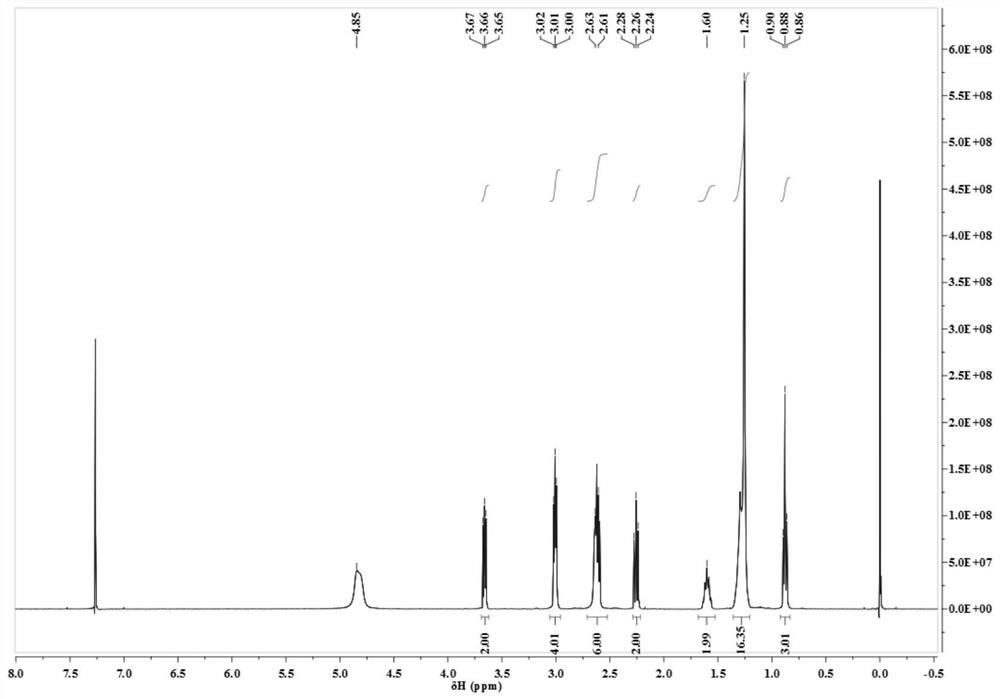
[0040] (1) Proton NMR spectrum
[0041] Using deuterated chloroform as a solvent, the proton nuclear magnetic resonance spectrum was measured on a Bruker DMX 500MHz nucle...
Embodiment 2
[0048] The synthetic method of the piperazine-based ionic liquid surfactant of the present embodiment is as follows:
[0049] (1) Add 0.05mol 1-(2-hydroxyethyl)piperazine and 30mL methanol into the round bottom flask;
[0050] (2) After dissolving 0.05mol of dodecanoic acid in 90mL of methanol, gradually add it to the methanol solution of 1-(2-hydroxyethyl)piperazine, keep the round bottom flask in an ice-water bath during this process, then stir at room temperature for 12h, Stirring rate 500rpm;
[0051] (3) Distilled under reduced pressure at room temperature, the resulting solid was washed with ethyl acetate, filtered, and then vacuum-dried at 40°C for 24 hours to obtain the product 1-(2-hydroxyethyl)piperazine dodecanoate with a yield of 94.3%.
[0052] Structural characterization of 1-(2-hydroxyethyl)piperazine dodecanoate:
[0053] (1) Proton NMR spectrum
[0054] With deuterated chloroform as solvent, measure proton nuclear magnetic resonance spectrum on Bruker DMX 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com