Carbon-carbon double bond bridging covalent organic framework material and preparation method thereof
A technology of covalent organic framework and carbon-carbon double bond is applied in the field of carbon-carbon double bond bridged covalent organic framework material and its preparation, which can solve the limited problems and achieve good thermal stability, high crystallinity, high ratio The effect of surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
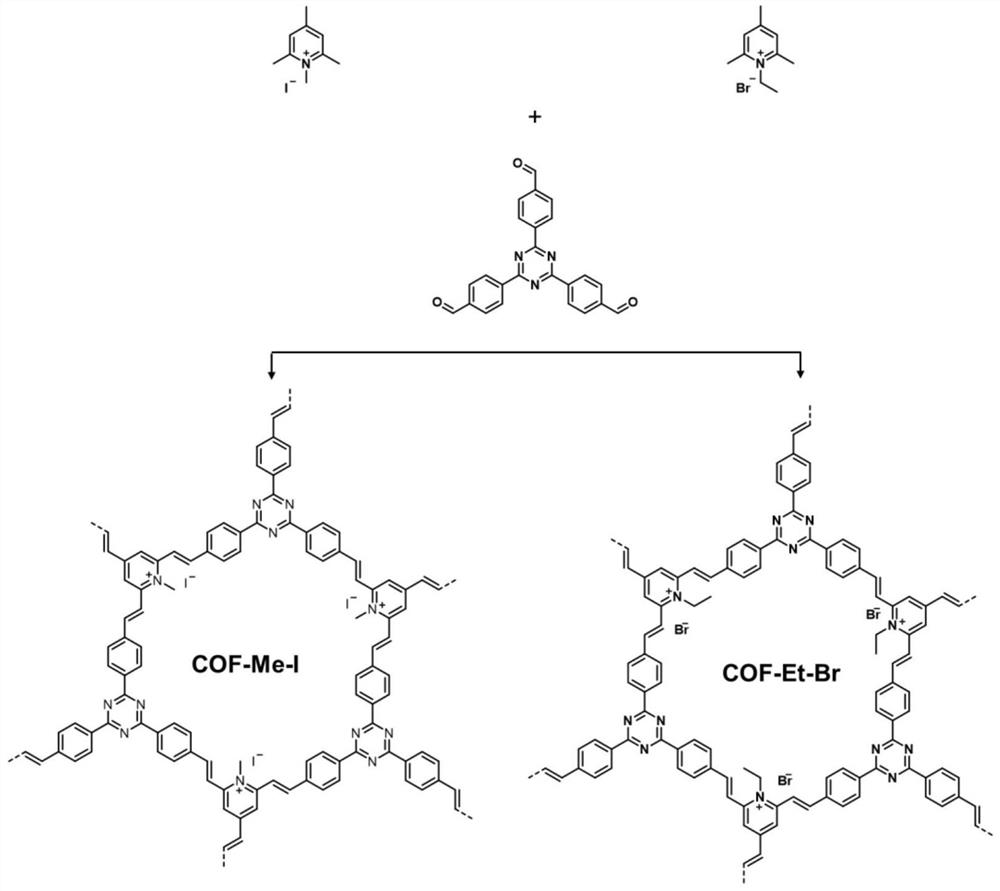
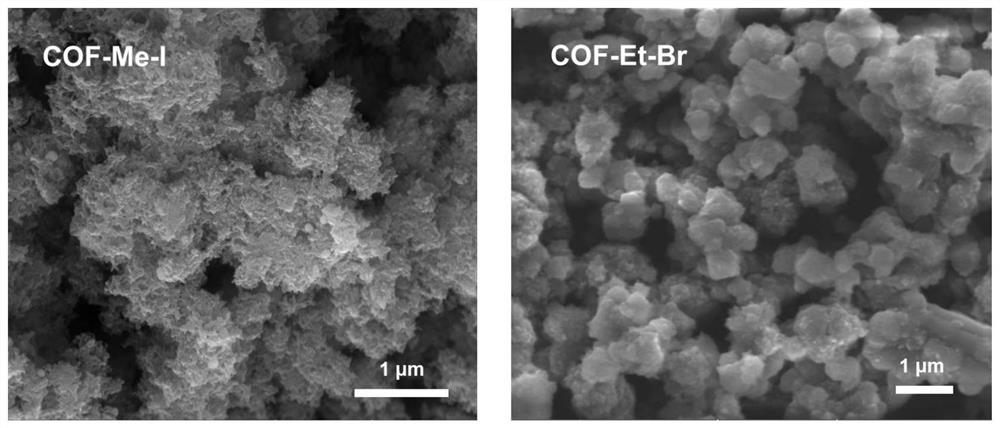
[0033] Such as figure 1 As shown, in an argon atmosphere glove box, 53 mg of N-methyl-2,4,6-trimethylpyridinium iodide salt, 79 mg of 2,4,6-tris(4-formylphenyl) -1,3,5-triazine, 7 mL of anhydrous N,N-dimethylformamide and 3 mL of o-dichlorobenzene and 600 μL of dimethylamine in tetrahydrofuran (2mol / L), add 15 mL of thick-walled in a pressure-resistant bottle. Seal the thick-walled pressure-resistant bottle with a polytetrafluoroethylene screw plug, transfer it to a constant temperature oil bath, and heat it to 180° C. for 72 hours. After the reaction, the reaction flask was naturally cooled to room temperature, and the filter residue was collected by vacuum filtration, rinsed with acetone, dichloromethane, tetrahydrofuran and methanol respectively, and the solid product was collected and vacuum-dried at 60° C. for 12 hours to obtain a light yellow solid. Named COF-Me-I.
Embodiment 2
[0035] Such as figure 1As shown, in an argon atmosphere glove box, 46 mg of N-ethyl-2,4,6-trimethylpyridinium bromide, 79 mg of 2,4,6-tris(4-formylphenyl) -1,3,5-triazine, 7Ml of anhydrous N,N-dimethylformamide and 3Md L of o-dichlorobenzene and 600μL of dimethylamine in tetrahydrofuran (2mol / L), add 15Ml of thick-walled in a pressure-resistant bottle. Seal the thick-walled pressure-resistant bottle with a polytetrafluoroethylene screw plug, transfer it to a constant temperature oil bath, and heat it to 180° C. for 72 hours. After the reaction, the reaction flask was naturally cooled to room temperature, and the filter residue was collected by vacuum filtration, rinsed with acetone, dichloromethane, tetrahydrofuran and methanol respectively, and the solid product was collected and vacuum-dried at 60° C. for 12 hours to obtain a light yellow solid. Named COF-Et-Br.
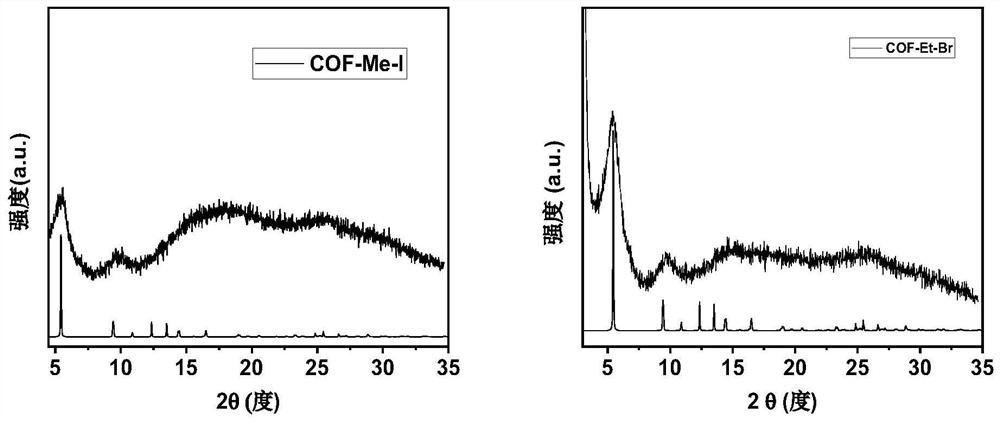
[0036] Such as figure 2 Shown is the powder X-ray diffraction pattern of the obtained two-dimensional catio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com