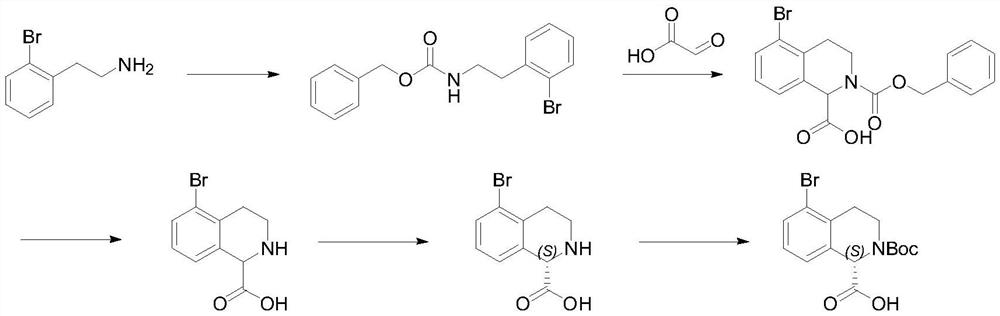
Preparation method of (S)-5-bromo-1, 2, 3, 4-tetrahydro-N-Boc-isoquinoline-1-carboxylic acid
A technology of tetrahydroisoquinoline and isoquinoline, which is applied in the fields of organic chemistry, organic chemistry, etc., can solve the problems of no synthetic method, etc., and achieve the effects of easy-to-obtain raw materials, mild reaction conditions, and reasonable and effective synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
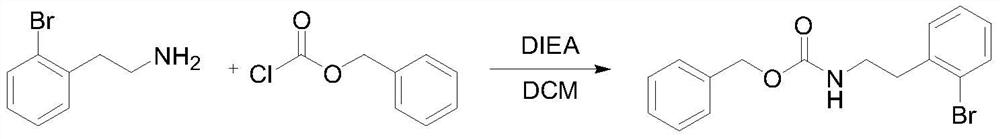
[0026] The first step: the synthesis of benzyl o-bromophenethylamine formate.
[0027]
[0028] Add o-bromophenethylamine (360g, 1.8mol), diisopropylethylamine (581.6g, 4.5mol) and 1800g of dichloromethane into a 3L jacketed reaction flask, lower the temperature and control it at -2-2°C, add dropwise Benzyl chloroformate (307g, 1.8mol), after dropping, slowly raise the temperature to 20°C, react for 2 hours, GC detects 0.5% of the raw material, add 0.2M hydrochloric acid to adjust pH=4, separate layers, wash the organic phase with water, and wash with saturated saline Once, the organic phase was concentrated until it was stagnant, and n-heptane was added to make a slurry to obtain 547.2 g of benzyl o-bromophenethylamine formate, with a yield of 91%, and GC: 97.3%. 1 HNMR (400MHz, CDCl 3 )δ:7.55-7.01(m,9H),4.75(s,1H),4.11(m,2H),3.48-3.40(m,2H),2.96(m,2H),1.23(m,3H).
[0029]
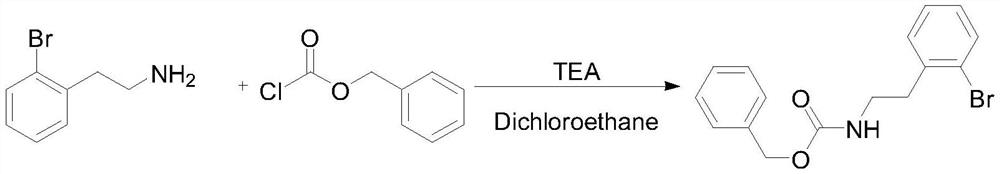
[0030] Add o-bromophenethylamine (360g, 1.8mol), triethylamine (400.7g, 3.96mol) and 1800g dic...
Embodiment 2
[0032] The second step: the synthesis of 5-bromo-2-(benzyloxycarbonyl)-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid.
[0033]
[0034] Add concentrated sulfuric acid / acetic acid = 1:3 (700mL), 50% glyoxylic acid (666.4g, 4.5mol) and 2.5L toluene into a 5L jacketed reaction flask, raise the temperature to 30-35°C, and slowly add o-bromophenethylamine Benzyl formate (501.3g, 1.5mol), dropwise, react at 30-35°C for 8 hours, HPLC detects 0.5% of the raw material, separate layers, extract the acidic aqueous layer with toluene once, combine the organic phases, adjust with sodium bicarbonate aqueous solution pH=2-3, separate layers, and concentrate the organic phase until stagnant to obtain 541.4g of 5-bromo-2-(benzyloxycarbonyl)-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid , yield 92.5%, GC: 94.9%. 1 HNMR (400MHz, CDCl 3 )δ:10.4(s,1H),7.55-7.01(m,8H),5.61(d,1H),4.26-3.86(m,3H),3.68-3.61(m,1H),2.95(m,2H) ,1.33-1.20(m,3H).
[0035]
[0036] Add glyoxylic acid (144.4g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com