Porous high-entropy alloy self-supporting electrode for electrolyzing water and preparation method thereof
A self-supporting electrode, high-entropy alloy technology, applied in the field of electrolysis of water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The invention provides a method for preparing a high-entropy alloy-based electrolytic water catalyst, comprising the following steps:
[0032] 1) Melting and mixing nickel powder, cobalt powder, chromium powder, iron powder, aluminum powder and tungsten powder (the molar ratio of which is 30:30:10:10:18:2), and cooling to form a eutectic structure (FCC phase + B2 phase) high-entropy alloy;
[0033] 2) Use a wire cutting machine to cut an area of 1×1cm from a large ingot 2 , a square sheet with a thickness of 400 μm, simply polished by a grinding and polishing machine to remove the surface oxide skin, and the thickness is about 300 μm at this time;
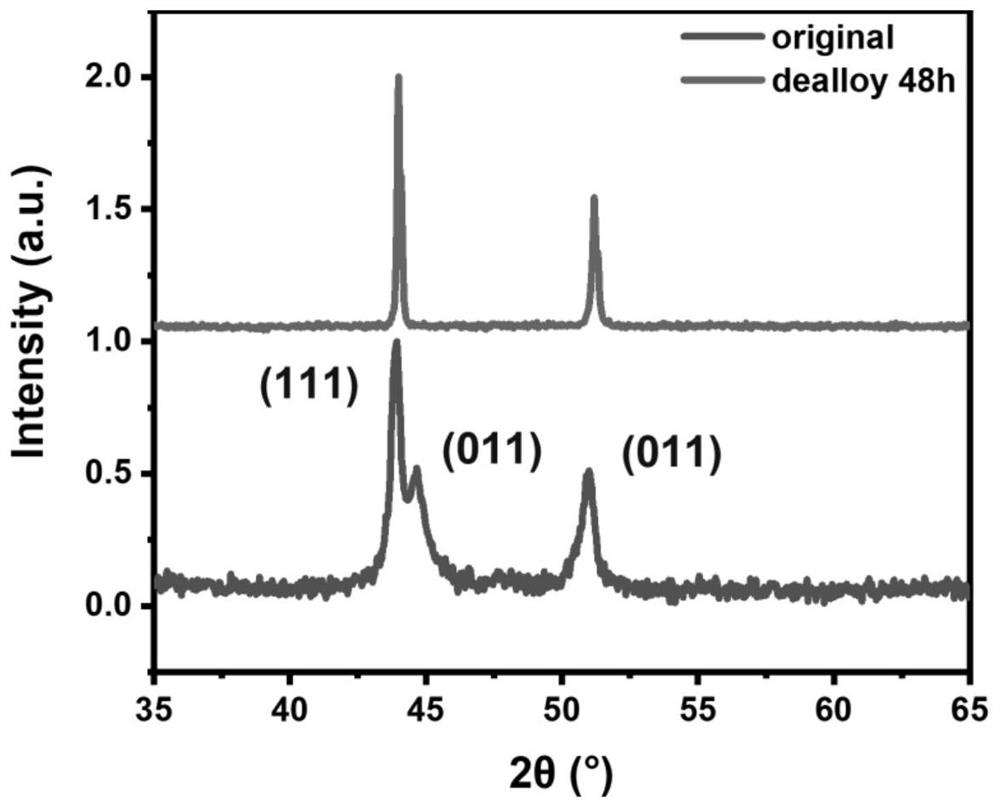
[0034] 3) Take 30mL of 1M HCl solution and pipette it into a 50mL centrifuge tube, put a piece of high-entropy alloy sheet into it, soak for 6, 12, 24, 48, 60, and 72h for dealloying treatment; the mass fraction of HCl solution is Anything below 20% is fine.
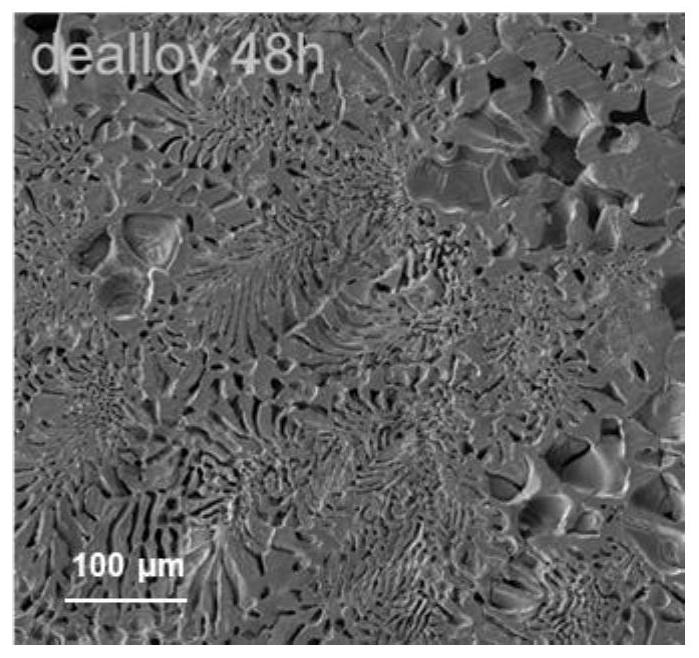
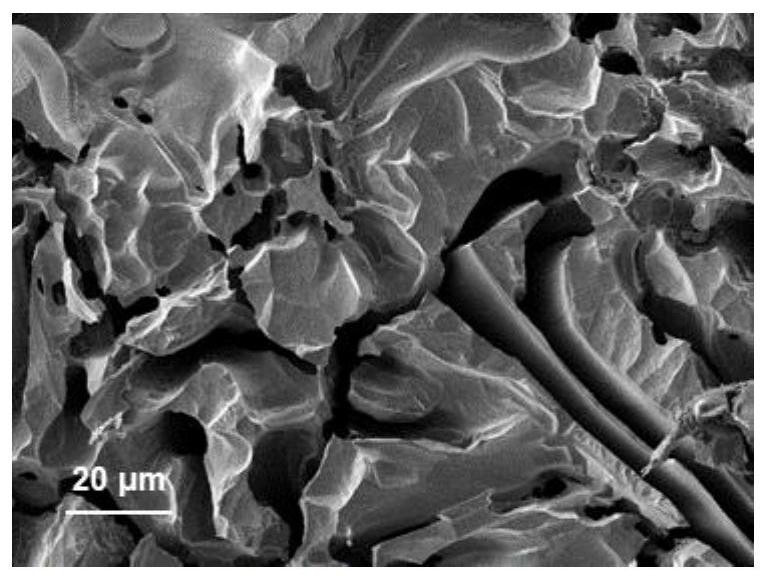
[0035] 4) After the hydrochloric acid dealloying treatment i...
Embodiment 1
[0038] Raw material components and dosage are as follows:
[0039] Nickel powder, cobalt powder, chromium powder, iron powder, aluminum powder, tungsten powder (the molar ratio is 30:30:10:10:18:2), hydrochloric acid solution (concentration is 1mol / L), potassium hydroxide solution ( The concentration is 1mol / L).
[0040] The preparation technology of this high-entropy alloy-based catalyst:
[0041] Melt and mix nickel powder, cobalt powder, chromium powder, iron powder, aluminum powder, tungsten powder (the molar ratio is 30:30:10:10:18:2), and form a two-phase eutectic structure (FCC) after cooling. phase + B2 phase) of high-entropy alloys; using a wire cutting machine to cut an area of 1 × 1cm from a large ingot 2 , a square sheet with a thickness of 400 μm, and the surface oxide skin was simply polished by a grinding and polishing machine, and the thickness was about 300 μm at this time; take 30mL of 1M HCl corrosion solution and pipette it into a 50mL centrifuge tube, ...
Embodiment 2
[0056] Raw material components and dosage are as follows:
[0057] Nickel powder, cobalt powder, chromium powder, iron powder, aluminum powder, tungsten powder (the molar ratio is 25:20:20:15:15:5), hydrochloric acid solution (concentration is 2mol / L), potassium hydroxide solution ( The concentration is 1mol / L).
[0058] The preparation technology of this high-entropy alloy-based catalyst:
[0059] Melt and mix nickel powder, cobalt powder, chromium powder, iron powder, aluminum powder, tungsten powder (the molar ratio is 25:20:20:15:15:5), and form a two-phase eutectic structure (FCC) after cooling phase + B2 phase) of high-entropy alloys; using a wire cutting machine to cut an area of 1 × 1cm from a large ingot 2 , a square sheet with a thickness of 400 μm, and the surface oxide scale was simply polished by a grinding and polishing machine, and the thickness was about 300 μm at this time; take 30mL of 2M HCl corrosion solution and pipette it into a 50mL centrifuge tube, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com