Preparation method of 2-amino-3-methyl-5-chlorobenzoic acid
A technology of methylbenzoic acid and chlorobenzoic acid, which is applied in the preparation of nitro compounds, organic compounds, and cyanide reaction preparations, and can solve problems such as large environmental pollution, low yield, and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
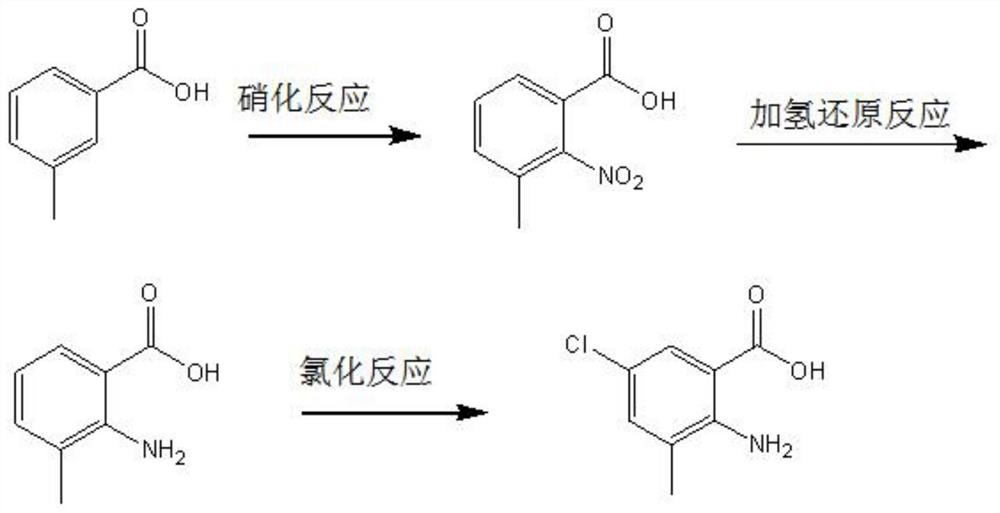
[0029] The invention provides a kind of preparation method of 2-amino-3-methyl-5-chlorobenzoic acid, comprising the following steps:
[0030] Mixing m-toluic acid and nitric acid for nitration reaction to obtain 2-nitro-3-methylbenzoic acid, the mass concentration of the nitric acid is 60% to 75%;
[0031] Mixing the 2-nitro-3-methylbenzoic acid, a hydrogenation reduction reaction solvent and a hydrogenation catalyst, and performing a hydrogenation reduction reaction in a hydrogen atmosphere to obtain 2-amino-3-methylbenzoic acid;
[0032] The 2-amino-3-methylbenzoic acid, chlorination reagent, benzoyl peroxide and chlorination reaction solvent are mixed, and the chlorination reaction is carried out to obtain 2-amino-3-methyl-5-chlorobenzoic acid .
[0033] In the present invention, unless otherwise specified, the raw materials used are commercially available products well known to those skilled in the art.
[0034] The present invention takes m-toluic acid as raw material, ...
Embodiment 1
[0057] Add 400ml of nitric acid with a mass concentration of 65% into a 1L four-neck glass flask, cool down to -10°C, add 100g (0.734mol) of m-toluic acid in batches, and keep stirring at 0°C for 1 hour after the addition is completed. Phase chromatography detects that the area-normalized content of m-toluic acid is less than 0.3% to end the reaction, filter the obtained nitration reaction system, rinse and dry the solid with 100ml of cold water to obtain 106.2g of 2-nitro-3-methylbenzoic acid , the purity of 2-nitro-3-methylbenzoic acid determined by liquid phase is 98.7%, the yield is 79.8%, and the melting point is 217-219°C.
[0058] Add 106.2g (0.586mol) of 2-nitro-3-methylbenzoic acid to a 1L four-necked flask, add 318.6ml of ethanol, stir to dissolve, add 5.3g of 10% Pd / C, connect the four-necked flask to the hydrogen bag Finally, pass hydrogen through vacuuming, and cycle for 3 times to ensure that the air is completely removed, then pass hydrogen, the pressure of hydr...
Embodiment 2
[0063]Add 800ml of nitric acid with a mass concentration of 65% into a 2L four-neck glass flask, cool down to -10°C, add 200g (1.469mol) of m-toluic acid in batches, and keep stirring at -10°C for 1 hour after the addition, and pass through the liquid phase Chromatographically detects that the area-normalized content of m-toluic acid is less than 0.3% to end the reaction, filter the obtained nitration reaction system, and obtain 210.4 g of 2-nitro-3-methylbenzoic acid after the solid is rinsed with 200 ml of cold water and dried. The purity of 2-nitro-3-methylbenzoic acid determined by liquid phase was 98.8%, the yield was 79.0%, and the melting point was 217-219°C.
[0064] Add 210.4g (1.161mol) 2-nitro-3-methylbenzoic acid to a 2L four-necked flask, add 631ml of ethanol, stir to dissolve, add 10.5g10%Pd / C, connect the four-necked flask to the hydrogen bag, After evacuating, hydrogen gas is circulated for 3 times to ensure that the air is completely removed, and then hydrogen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com