Method for detecting antioxidant degradation product in sodium chloride injection of levofloxacin lactate
A technology of levofloxacin lactate and sodium chloride injection, which can be applied to measurement devices, instruments, scientific instruments and other directions, and can solve the problems of inability to detect antioxidant degradation products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
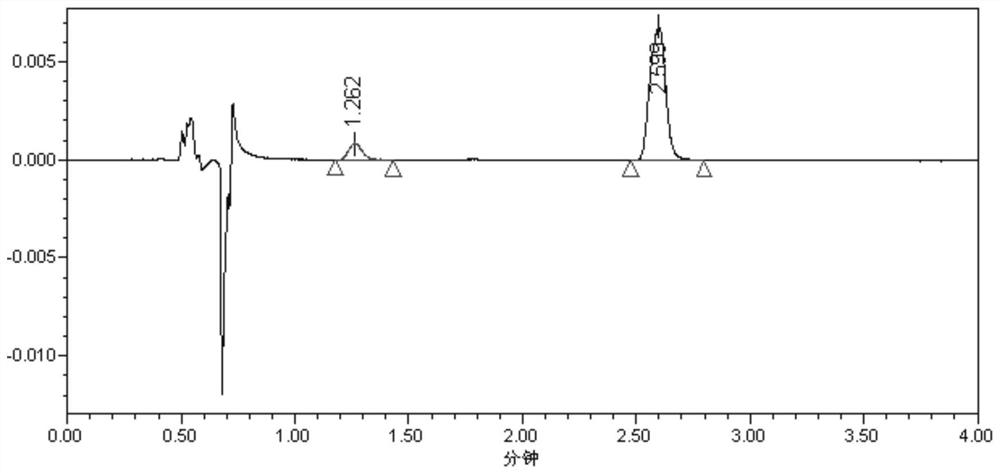
[0042] Preparation of reference substance solution: Mix 2,4-di-tert-butylphenol and 3,5-di-tert-butyl-4-hydroxyphenylpropionic acid reference substance, and prepare with solvent (tetrahydrofuran and methanol at a volume ratio of 1:1) The concentration of 2,4-di-tert-butylphenol was 6.7 μg / mL, and the concentration of 3,5-di-tert-butyl-4-hydroxyphenylpropionic acid was 0.8 μg / mL.
[0043] The HPLC detection conditions of antioxidant degradation products 2,4-di-tert-butylphenol and 3,5-di-tert-butyl-4-hydroxyphenylpropionic acid are:
[0044] Detector: UV detector;
[0045] Detection wavelength: 280nm;
[0046] Chromatographic column: ACQUITY UPLC BEH C18, 2.1*100mm, 1.7μm;
[0047] Mobile phase: acetonitrile-water solution with a volume ratio of 70:30;
[0048] Flow rate: 0.4mL / min;
[0049] Column temperature: 30°C;
[0050] Injection volume: 3μL;
[0051] The elution method is isocratic elution.
[0052] Get the reference substance solution of above-mentioned preparation...
Embodiment 2
[0069] Preparation of reference substance solution: Mix 2,4-di-tert-butylphenol and 3,5-di-tert-butyl-4-hydroxyphenylpropionic acid reference substance, and prepare with solvent (tetrahydrofuran and methanol at a volume ratio of 1:1) The concentration of 2,4-di-tert-butylphenol was 6.7 μg / mL, and the concentration of 3,5-di-tert-butyl-4-hydroxyphenylpropionic acid was 0.8 μg / mL.
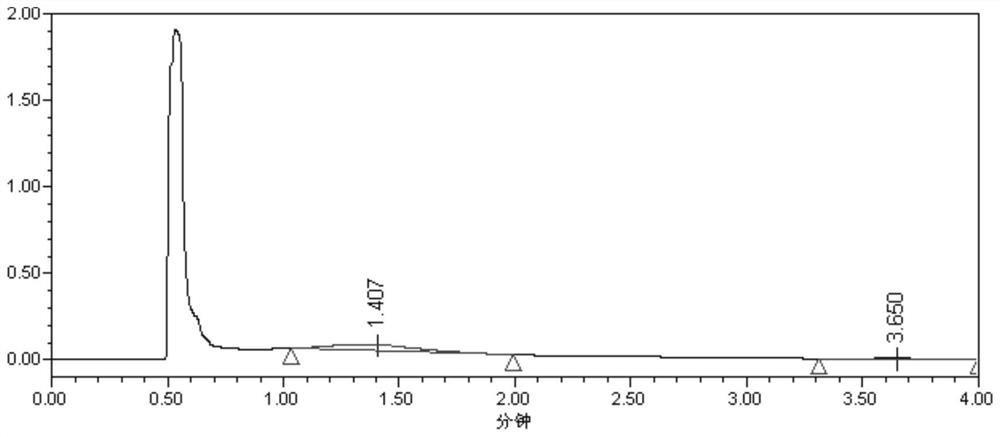
[0070] Preparation of the test solution: after taking 50 mL of levofloxacin lactate sodium chloride injection and passing through a solid phase extraction column (Supel-Select HLB SPE, 6 mL), add 5 mL of 0.002 mol / L acetic acid solution, then use 6 mL of methanol and 4 mL of tetrahydrofuran successively For elution, collect the eluent, and use a solvent (tetrahydrofuran and methanol at a volume ratio of 1:1) to set the volume to 10 mL, filter, and use it as the test solution.
[0071] Preparation of the sample addition solution: take 50 mL of levofloxacin lactate sodium chloride injection, accurately...
Embodiment 3
[0111] Cleaning method recovery confirmation:
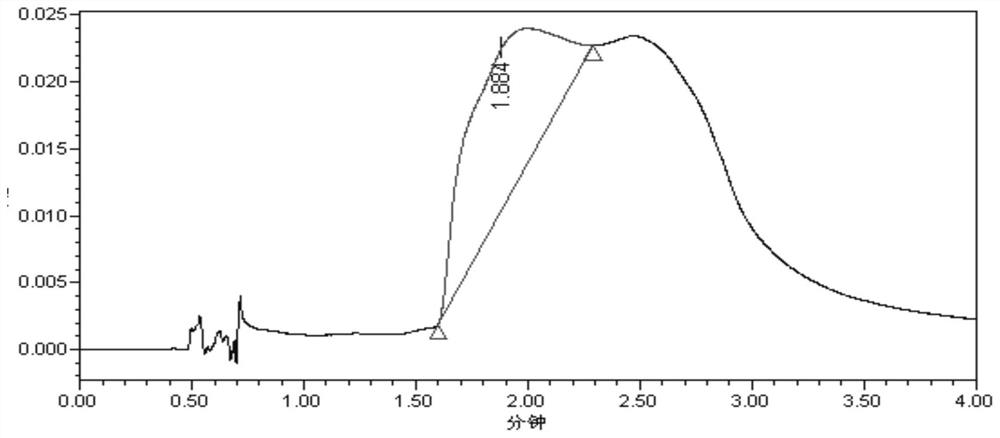
[0112] Preparation of reference substance solution: Mix 2,4-di-tert-butylphenol and 3,5-di-tert-butyl-4-hydroxyphenylpropionic acid reference substance, and prepare with solvent (tetrahydrofuran and methanol at a volume ratio of 1:1) The concentration of 2,4-di-tert-butylphenol was 6.7 μg / mL, and the concentration of 3,5-di-tert-butyl-4-hydroxyphenylpropionic acid was 0.8 μg / mL.
[0113] The preparation of the test solution: after taking 50 mL of levofloxacin lactate sodium chloride injection through the activated solid phase extraction column (Supel-Select HLB SPE, 6 mL), add 0.002 mol / L acetic acid solution 5 mL, then use 6 mL methanol and 4mL tetrahydrofuran was eluted sequentially, and the eluate was collected, and the volume was adjusted to 10mL with a solvent (tetrahydrofuran and methanol at a volume ratio of 1:1) to obtain a stock solution for future use.
[0114] Precisely measure 1mL of the reference substance solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com