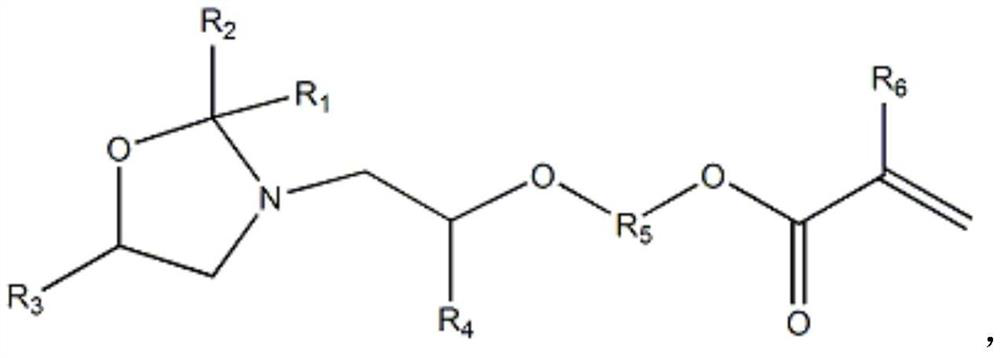
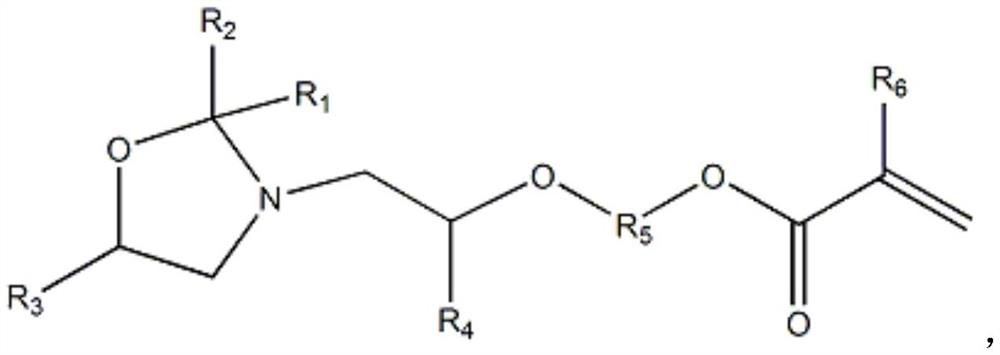
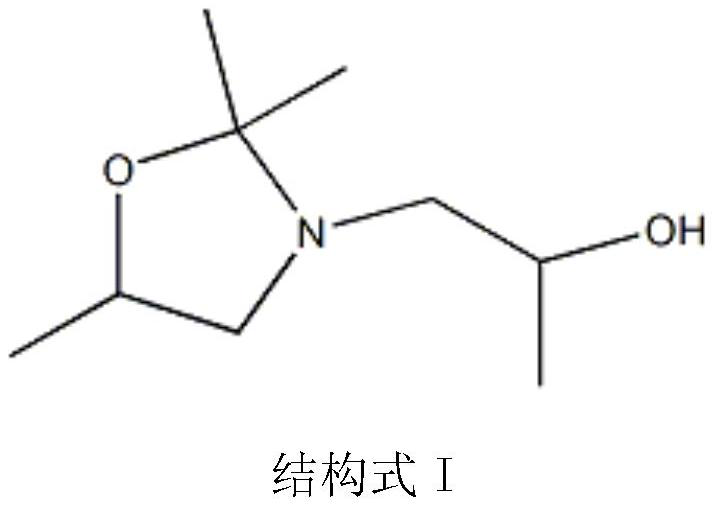
Oxazolidine-containing radiation-curable urethane(meth)acrylate and preparation method thereof
A radiation curing and acrylate technology, applied in organic chemistry, coatings, epoxy resin coatings, etc., can solve problems such as the inability to ensure the adhesion, hardness, flexibility, and high viscosity of the coating film at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of cyclic oxazolidine monoalcohols from diisopropanolamine and acetone:
[0043] Add (134g, 1mol) diisopropanolamine, (75.4g, 1.3mol) acetone, (4.11g, 30mmol) 70% methanesulfonic acid and 98.9g cyclohexane into a condenser equipped with electric stirring and water-cooled reflux In a 1000ml reaction flask, place the flask in a sand bath in an electric heating mantle, use a temperature controller connected to a thermocouple immersed in the sand bath to control the temperature of the sand bath, and heat the solution in the flask to 80°C with stirring in the sand bath , the reaction temperature was maintained at 80°C and continued to stir for 10 hours. During the reaction, the water produced by the reaction was continuously distilled out by the cyclohexane solvent, and the distilled cyclohexane and acetone were returned to the reaction flask to continue the reaction. After the reaction, the excess acetone and cyclohexane The hexane solvent was distilled out of t...
Embodiment 2
[0047] Preparation of cyclic oxazolidine monoalcohols from bis(4-hydroxypentyl)amine and 2-hexanone:
[0048] (190g, 1mol) two (4-hydroxypentyl) amine, (130g, 1.3mol) 2-hexanone, (4.11g, 30mmol) 70% methanesulfonic acid and 147.5g cyclohexane were added to the motor In a 1000 ml reaction flask with stirring and a water-cooled reflux condenser, place the flask in a sand bath in a heating mantle, control the temperature of the sand bath using a temperature controller connected to a thermocouple immersed in the sand bath, and heat the flask under stirring using the sand bath The content of the inner solution reaches 80°C, the reaction temperature is maintained at 80°C and continues to stir for 10 hours. During the reaction, the water generated by the reaction is continuously distilled out by the cyclohexane solvent, and the distilled cyclohexane and hexanone are returned to the reaction flask to continue the reaction. Excessive hexanone and cyclohexane solvents were distilled out...
Embodiment 3
[0051] Preparation of Cyclic Oxazolidine Monoalcohols from Diisopropanolamine and Butanone:
[0052] Add (134g, 1mol) diisopropanolamine, (93.6g, 1.3mol) butanone, (4.11g, 30mmol) 70% methanesulfonic acid and 105g cyclohexane into a condenser equipped with electric stirring and water-cooled reflux In a 1000ml reaction flask, place the flask in a sand bath in an electric heating mantle, use a temperature controller connected to a thermocouple immersed in the sand bath to control the temperature of the sand bath, and heat the solution in the flask to 80°C with stirring in the sand bath , the reaction temperature was maintained at 80°C and continued to stir for 10 hours. During the reaction, the water produced by the reaction was continuously distilled out by the cyclohexane solvent, and the distilled cyclohexane and butanone were refluxed into the reaction flask to continue the reaction. After the reaction, the excess butanone And the cyclohexane solvent was distilled out of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com