Raman sensing analysis method for detecting enterotoxin
An analysis method, enterotoxin technology, applied in the field of optical analysis, to achieve the effect of enhancing SERS signal, realizing detection and improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
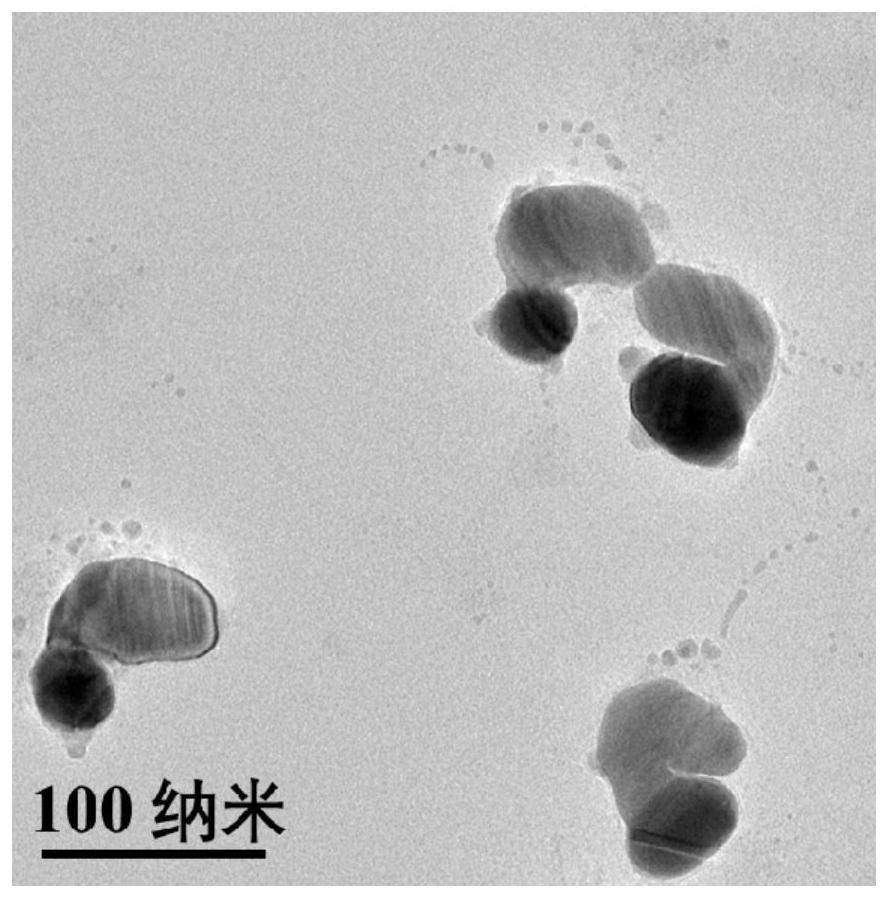
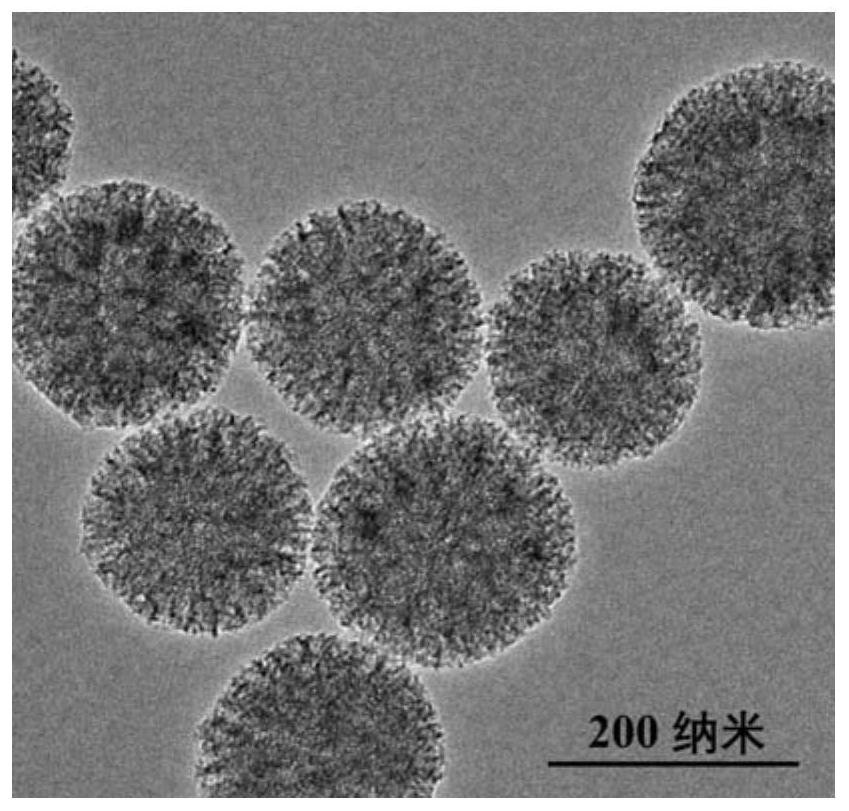
[0031] 1. Preparation of plasmonic nanomaterial Au-Ag Janus NPs:
[0032] ① Preparation of monodisperse Au NPs:
[0033] Au NPs were prepared as seeds by citric acid reduction method. 0.8mL HAuCl 4 Mix with ultrapure water and heat to boiling. Quickly add 0.9mL sodium citrate solution under vigorous stirring, and the mixed solution continues to boil for 15min. Then cool down to room temperature naturally, and centrifuge and wash with ultrapure water to obtain Au NPs;
[0034] ② Preparation of Au-Ag Janus NPs:
[0035] Under vigorous stirring, 20 μL of 0.8 mM 2-mercaptobenzimidazole-5-carboxylic acid (MBIA) was added to 1 mL of citric acid-stabilized Au NPs synthesized in step (1). The mixed solution was incubated in a water bath at 60°C for 2h. After the reaction, the solution was cooled to room temperature, and 50 μL of 8 mM hydroquinone (HQ) and AgNO at a final concentration of 50 μM were added at a constant speed under vigorous stirring. 3 solution. The resulting mi...
Embodiment 2
[0046] 1. Preparation of plasmonic nanomaterial Au-Ag Janus NPs:
[0047] ① Preparation of monodisperse Au NPs:
[0048] Au NPs were prepared as seeds by citric acid reduction method. 1.0mL HAuCl 4 Mix with ultrapure water and heat to boiling. Quickly add 1.0mL sodium citrate solution under vigorous stirring, and the mixed solution continues to boil for 17.5min. Then cool down to room temperature naturally, and centrifuge and wash with ultrapure water to obtain Au NPs;
[0049] ② Preparation of Au-Ag Janus NPs:
[0050] Under vigorous stirring, 22 μL of 1 mM 2-mercaptobenzimidazole-5-carboxylic acid (MBIA) was added to 1.1 mL of citric acid-stabilized Au NPs synthesized in step (1). The mixed solution was incubated in a water bath at 65°C for 2.5h. After the reaction, the solution was cooled to room temperature, and 60 μL of 10 mM hydroquinone (HQ) and AgNO with a final concentration of 52.5 μM were added at a constant speed under vigorous stirring. 3 solution. The res...
Embodiment 3
[0061] 1. Preparation of plasmonic nanomaterial Au-Ag Janus NPs:
[0062] ① Preparation of monodisperse Au NPs:
[0063] Au NPs were prepared as seeds by citric acid reduction method. 1.2mL HAuCl 4 Mix with ultrapure water and heat to boiling. Quickly add 1.1mL sodium citrate solution under vigorous stirring, and the mixed solution continues to boil for 20min. Then cool down to room temperature naturally, and centrifuge and wash with ultrapure water to obtain Au NPs;
[0064] ② Preparation of Au-Ag Janus NPs:
[0065] Under vigorous stirring, 24 μL of 1.2 mM 2-mercaptobenzimidazole-5-carboxylic acid (MBIA) was added to 1.2 mL of citric acid-stabilized Au NPs synthesized in step (1). The mixed solution was incubated in a water bath at 70°C for 3h. After the reaction, the solution was cooled to room temperature, and 70 μL of 12 mM hydroquinone (HQ) and AgNO with a final concentration of 55 μM were added at a constant speed under vigorous stirring. 3 solution. The resulti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com