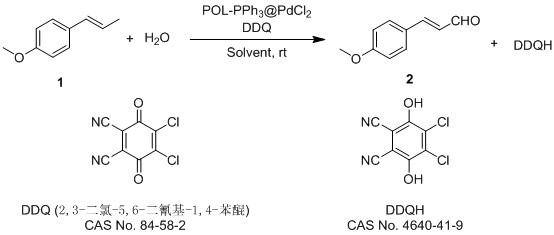
Method for preparing p-methoxycinnamyl aldehyde spice by using anethole
A technology of methoxycinnamaldehyde and anethole, applied in the field of flavors and fragrances, can solve the problems of only 25% yield, difficult extraction, high price, etc., and achieve high production efficiency, high selectivity, and production low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] POL-PPh used in the present invention 3 The preparation method is as follows: 1.0g 3V-PPh 3 Dissolve in 10ml of THF, then add 25mg of azobisisobutyronitrile, mix evenly, transfer to a reaction kettle, react at 100°C for 24 hours, and evaporate the solvent in vacuo to obtain white powder POL-PPh3.
[0029] The 3V-PPh 3 The preparation process is as follows: 22mmol of PPh 3 Dissolve it in 30ml THF, and slowly drop it into 4-vinylmagnesium bromide Grignard reagent at 0°C. After the addition is completed, continue the reaction at room temperature for 2 hours. 4 Quench the reaction with Cl solution, extract with excess ethyl acetate, wash the organic phase with saturated brine, dry over anhydrous magnesium sulfate, and finally purify the product with a silica gel column to obtain white 3V-PPh 3 solid.
Embodiment 1
[0031] A kind of method utilizing anethole to prepare p-methoxycinnamaldehyde perfume, comprises the steps:
[0032] (1) Catalyst preparation: under argon atmosphere, 500 mgPOL-PPh 3 , 25 mg PdCl 2 Add it to a 25 mL sealed tube, then add 5 mL of anhydrous THF, react overnight at 25°C, filter, remove the filtrate, wash the filter residue twice with ethyl acetate and ethanol, and collect the solid to obtain POL-PPh 3 @PdCl 2 Catalyst 518 mg;
[0033] (2) Catalytic oxidation reaction: weigh 100 mg POL-PPh in a 250 mL round bottom flask 3 @PdCl 2, Add 10 mmol (1.482 g) of anethole, 50 mmol (0.9 g) of water and 50 mL of toluene, stir well, then slowly add 20 mmol of DDQ (4.54 g) into the round bottom flask, and catalyze the oxidation reaction at room temperature for 1 h;
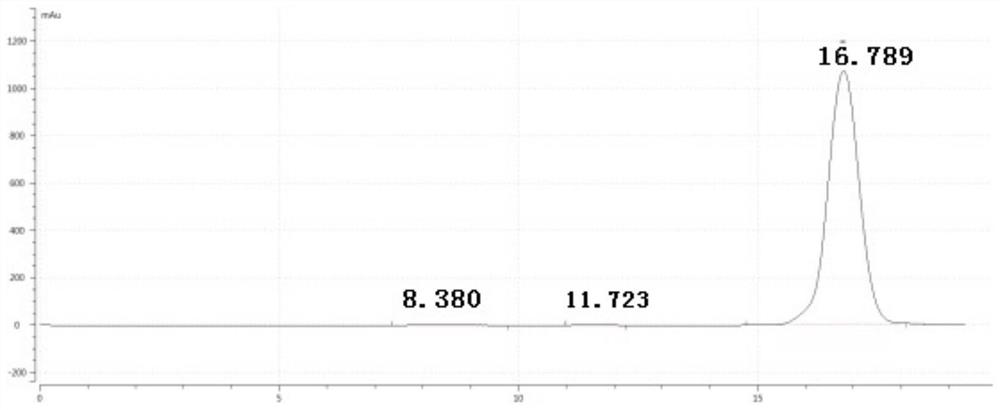
[0034] (3) Purification: filter the reaction liquid obtained in step (2) through diatomaceous earth, add 20mL water for extraction, and use anhydrous Na 2 SO 4 Dry, concentrate in vacuo to recover the orga...
Embodiment 2
[0037] A kind of method utilizing anethole to prepare p-methoxycinnamaldehyde perfume, comprises the steps:
[0038] (4) Catalyst preparation: under argon atmosphere, 500 mgPOL-PPh 3 , 40mg PdCl 2Add it to a 25 mL sealed tube, then add 5 mL of anhydrous THF, react overnight at 30°C, filter, remove the filtrate, wash the filter residue twice with ethyl acetate and ethanol, and collect the solid to obtain POL-PPh 3 @PdCl 2 Catalyst 532 mg;
[0039] (5) Catalytic oxidation reaction: weigh 100 mg POL-PPh in a 250 mL round bottom flask 3 @PdCl 2, Add 15mmol (2.223 g) of anethole, 45mmol (0.81 g) of water and 50mL of toluene, stir well, then slowly add 30mmol of DDQ (6.81g) into the round bottom flask, and catalyze the oxidation reaction at room temperature for 1.5h;
[0040] (6) Purification: filter the reaction solution obtained in step (2) through diatomaceous earth, add 30mL water for extraction, and use anhydrous Na 2 SO 4 Dry, concentrate in vacuo to recover the organic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com