Lignin grafted polyamino acid type heavy metal adsorbent as well as preparation method and application thereof
A polyamino acid and lignin technology, applied in separation methods, chemical instruments and methods, adsorbed water/sewage treatment, etc., can solve the problems of inability to adsorb heavy metal cations, waste of resources, poor repeatability of adsorbents, etc. The effect of short recovery and removal time and mild adsorption treatment conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~2
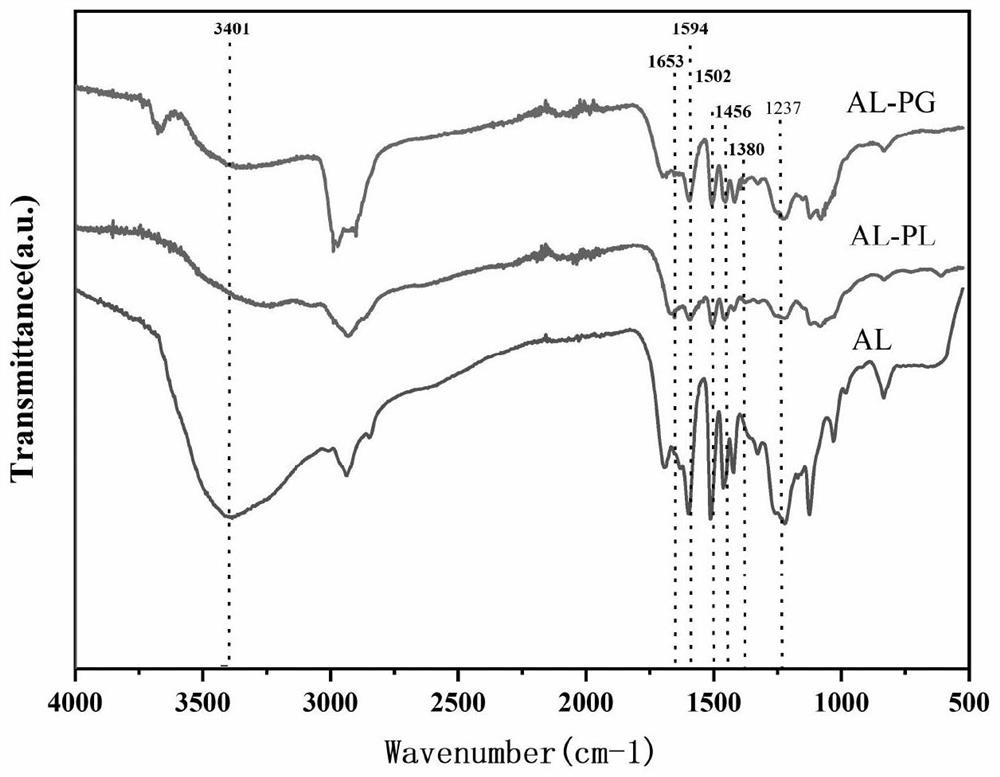
[0060] Step 1: Add 5g of lignin powder into water to prepare an aqueous solution with a mass fraction of 30%, add 0.2mol / L NaOH aqueous solution to adjust the pH to 12.0, and stir at the same time to completely dissolve the lignin. Add polylysine (2.5g, embodiment 1) and polyaspartic acid (4g, embodiment 2) respectively in the round bottom flask then, drop 2.5g of 37wt% formaldehyde solution in 20 minutes, be warming up to Reaction at 70°C for 3 hours; after the reaction, the reaction solution was subjected to nanofiltration with a nanofiltration membrane with a molecular weight cut-off of 1000 Da, and the pH at the end point of the nanofiltration was 5 to 7, and the concentrated solution after nanofiltration was freeze-dried to obtain polylysine respectively The N contents of modified lignin (AL-PL) and polyaspartic acid modified lignin (AL-PC) were 10.24% and 8.76% respectively according to elemental analysis. .
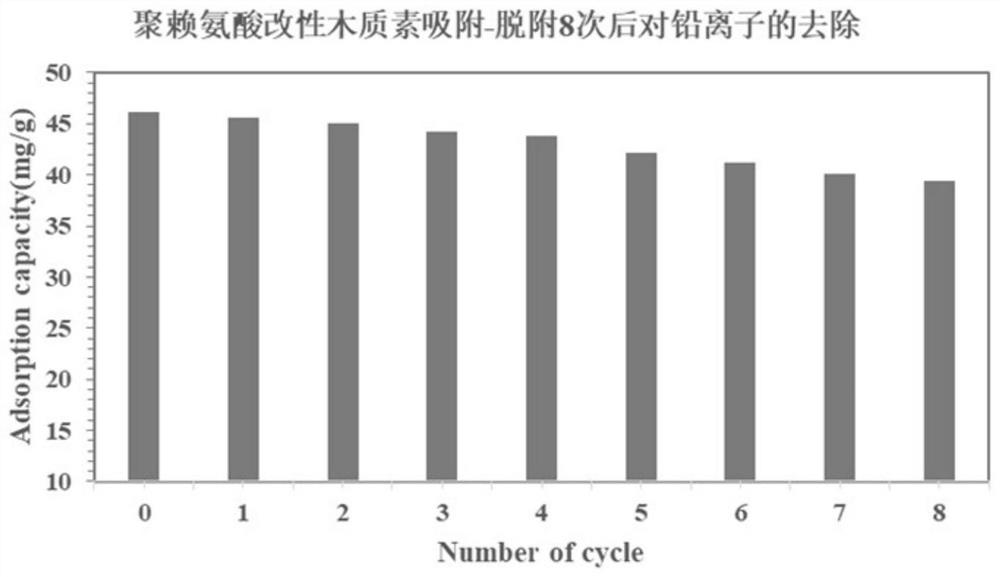
[0061] Step 2: Using AL-PL and AL-PC synthesized in Step 1 a...
Embodiment 3~4
[0074] Step 1: 5g of lignin powder was added into water to form an aqueous solution with a mass fraction of 25%, and 0.1mol / L NaOH aqueous solution was added to adjust the pH to 11.0, while stirring to completely dissolve lignin. Add polyglutamic acid (5g, embodiment 3) and polyarginine (7.5g, embodiment 4) respectively in the round-bottomed flask then, in 20min, 2.5g dropwise addition of 37wt% glyoxal solution finishes, is warming up to React at 60°C for 3 hours; after the reaction, the reaction solution is nanofiltered with a nanofiltration membrane with a molecular weight cut-off of 1000Da, and the pH at the end point of the nanofiltration is 5-7. The concentrated solution after nanofiltration is freeze-dried to obtain polyglutamic acid Natural lignin (AL-PG) and polyarginine-modified lignin (AL-PS), the N content of which was 6.21% and 8.32% by elemental analysis.
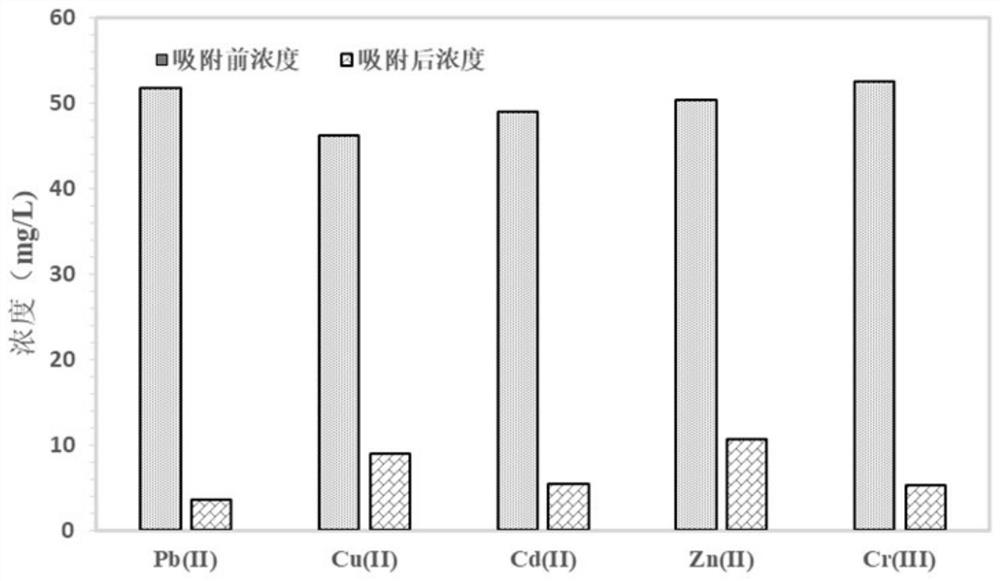
[0075] Step 2: Use AL-PG and AL-PS synthesized in Step 1 as adsorbents to adsorb and treat metal ions in the...
Embodiment 5~6
[0083]Step 1: 5g of lignin powder was added into water to form an aqueous solution with a mass fraction of 20%, and 0.4mol / L NaOH aqueous solution was added to adjust the pH to 11.0 while stirring to completely dissolve lignin. Add polyhistidine (6g, embodiment 5) and polyglycine (3g, embodiment 6) respectively in the round-bottomed flask then, in 20min, 2.5g of 37wt% glutaraldehyde solution is added dropwise, be warming up to 60 ℃ of reaction 2h; After the reaction, the reaction solution is nanofiltered with a nanofiltration membrane with a molecular weight cut-off of 1000Da, and the pH of the nanofiltration end point is 5-7. The concentrated solution after nanofiltration is freeze-dried to obtain polyhistidine-modified lignin (AL-PH) and polyglycine modified lignin (AL-PA), wherein the elemental analysis test its N content was 16.10% and 9.65%, respectively.
[0084] Step 2: Use AL-PH or AL-PA synthesized in Step 1 as an adsorbent to adsorb and treat metal ions in water. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Maximum adsorption capacity | aaaaa | aaaaa |
| Maximum adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com