Recombinant aspartate lyase and method for preparing R-3-aminobutyric acid with high repeated utilization rate
A technology of aspartic acid and aminobutyric acid, applied in the fields of enzyme engineering and biocatalysis, can solve the problems of inaccessibility, destruction of active centers, inactivation and denaturation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Construction of recombinant aspartate lyase expression strain
[0033] A recombinant aspartate lyase, which has an amino acid sequence formed by sequentially concatenating SEQ ID NO.2, SEQ ID NO.1, and SEQ ID NO.3. The amino acid sequence of SEQ ID NO.2 is TCRKSYHKQGNRYQTYSRCKH; SEQ ID NO. The amino acid sequence of 3 is CHTSYGRYRKQRK. The nucleotide coding sequence of the entire amino acid sequence is shown in SEQ ID NO.4.
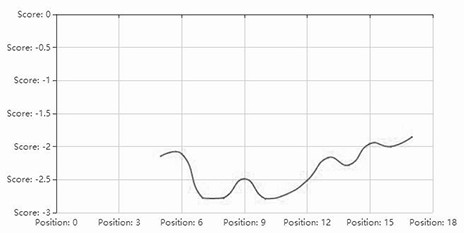
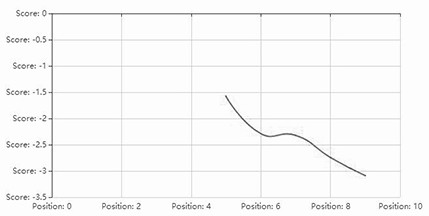
[0034] The hydrophilic distribution of the sequence SEQ ID NO.2 is as follows figure 1As shown in , the ordinate indicates the size of hydrophobicity (positive value indicates hydrophobicity, negative value indicates hydrophilicity); figure 2 The hydrophilic distribution of SEQ ID NO.3 is shown, and it can be seen that the proportion of polar amino acid residues is relatively large, and it has higher salt tolerance.
[0035] Entrust Shanghai Jierui Bioengineering Co., Ltd. to synthesize the gene, carry out double digestion and purification...
Embodiment 2
[0045] In this example, appropriate changes were made on the basis of Example 1, and the amino resin LX-1000HAA was replaced with epoxy resin LX-1000EP (Xi'an Lanxiao Technology New Materials Co., Ltd.), to obtain immobilized enzyme B.
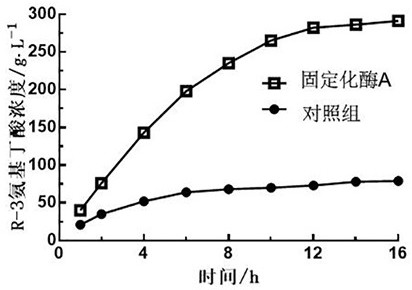
[0046] If the enzyme solution disclosed in ZL 201810198044.8 is used to immobilize with LX-1000EP, the recovery rate of enzyme activity is 4.8%. The immobilized enzyme B in this example was immobilized with the carrier and reused 20 times, and the conversion rate was above 98%.
Embodiment 3
[0048] In this example, appropriate changes were made on the basis of Example 1, and the amino resin LX-1000HAA was replaced with epoxy resin LX-103B (Xi'an Lanxiao Technology New Materials Co., Ltd.) to obtain immobilized enzyme C.
[0049] For example, using the enzyme solution disclosed in ZL 201810198044.8 and LX-103B for immobilization, the recovery rate of enzyme activity is 5.3%. The immobilized enzyme C in this example was immobilized with the carrier and reused 20 times, and the conversion rate was above 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com