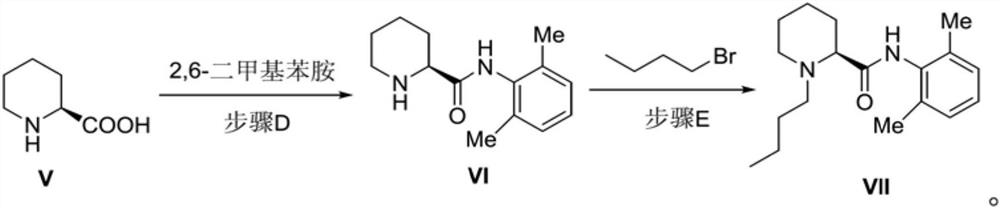
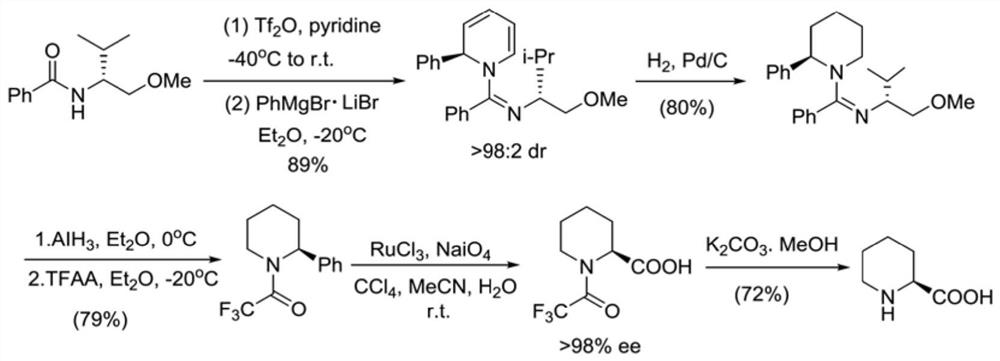
Preparation method of bupivacaine and intermediate (S)-2-piperidinecarboxylic acid thereof
A technology of piperidinecarboxylic acid and lithium hexamethyldisilazide, which is applied in the field of preparation of bupivacaine and its intermediate - 2-piperidinecarboxylic acid, can solve the problems of difficulty in obtaining starting materials, high production costs, and steps cumbersome and other problems, to achieve good market application prospects, low production costs, and good stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
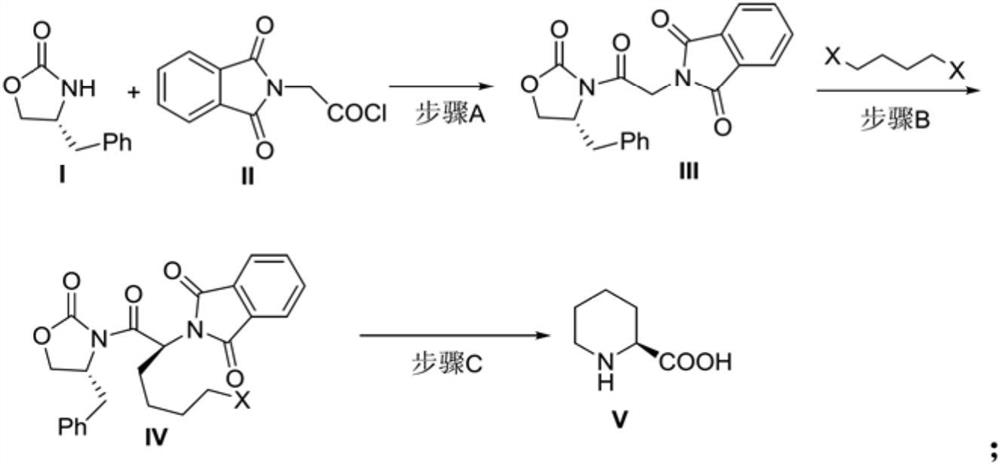
[0055] Embodiment 1, the synthesis of compound (III)
[0056] Dissolve (R)-4-benzyl-2-oxazolidinone I (1.62g, 16mmol) in tetrahydrofuran (20mL), and add n-butyllithium in n-hexane (7.7mL , 19.2mmol, 2.5mol / L), stirred at -78°C for 30 minutes, then added compound II (3.92g, 17.6mmol) in tetrahydrofuran (25mL), continued the reaction at this temperature for 30 minutes, then naturally rose to room temperature for reaction 3 hours. After the reaction was completed, 2 mL of glacial acetic acid was added dropwise to quench the reaction, then 30 mL of water was added and stirred, extracted with dichloromethane (2×100 mL), the organic phase was collected, dried, concentrated, and the crude product was purified by silica gel column chromatography, and the eluent was petroleum Ether: ethyl acetate (volume ratio: 5:1-3:1), after concentration, 5.3 g of light yellow oily liquid Compound III was obtained, with a yield of 91%.
[0057] 1 H NMR (500MHz, Chloroform-d) δ7.92-7.74(m, 4H), 7....
Embodiment 2
[0058] Embodiment 2, the synthesis of compound (III)
[0059] (R)-4-Benzyl-2-oxazolidinone I (1 g, 10 mmol) was dissolved in tetrahydrofuran (10 mL), and sodium hydride (480 mg, 12 mmol, content 60%) was added at -5°C under nitrogen protection, After stirring at 0° C. for 1 h, a solution of compound II (2.45 g, 11 mmol) in tetrahydrofuran (15 mL) was added, and then allowed to rise to room temperature for 5 hours. After the reaction, add 1 mL of glacial acetic acid dropwise to quench the reaction, then add 20 mL of water and stir, dichloromethane extraction (2 × 80 mL), collect the organic phase, dry, concentrate, and the crude product is purified by silica gel column chromatography, and the eluent is petroleum Ether: ethyl acetate (volume ratio: 5:1-3:1), after concentration, 3.13 g of light yellow oily liquid Compound III was obtained, with a yield of 86%.
Embodiment 3
[0060] Embodiment 3, the synthesis of compound (III)
[0061] Dissolve (R)-4-benzyl-2-oxazolidinone I (1g, 10mmol) in tetrahydrofuran (10mL), add potassium tert-butoxide (1.35g, 12mmol) at 0°C under nitrogen protection, and at this temperature After stirring for 1 h, a solution of compound II (2.45 g, 11 mmol) in tetrahydrofuran (15 mL) was added, and then allowed to rise to room temperature for 5 hours. After the reaction was finished, 20 mL of water was added to quench the reaction, extracted with dichloromethane (2 × 80 mL), the organic phase was collected, dried, concentrated, and the crude product was purified by silica gel column chromatography, and the eluent was sherwood oil: ethyl acetate (volume ratio 5:1~3:1), concentrated to obtain light yellow oily liquid compound III3g with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com