Preparation method of cyclic carbonate
A cyclic carbonate, alkylene oxide technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problem of low efficiency and unfavorable industrialization of cyclic carbonate preparation process The problems of high production and energy consumption can achieve good industrial application prospects, improve production safety, and stabilize quality parameters.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
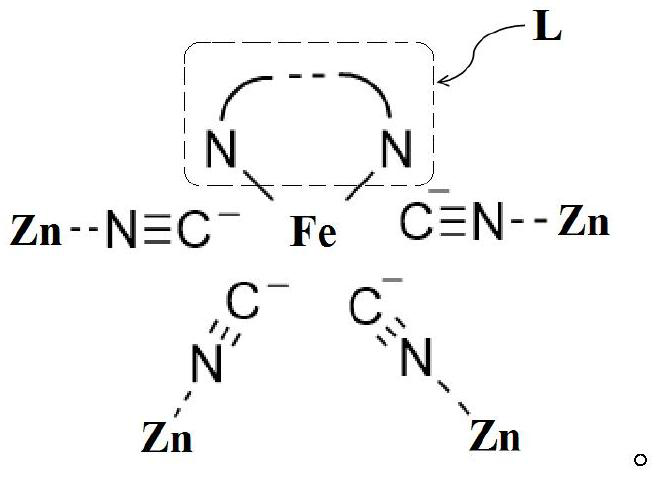
[0024] The invention provides a method for preparing a cyclic carbonate, comprising: using alkylene oxide and carbon dioxide as raw materials, contacting the raw material with a complex catalyst to obtain a cyclic carbonate; wherein, the general chemical formula of the complex catalyst is as follows:
[0025] (ZnX)·[Fe(CN) 4 ·mL]·(ZnX 2 ) n Wherein, m is 1 or 1 / 2, the value of n is selected from 1 to 40, preferably 1 to 10, and X represents F, Cl, Br or I;
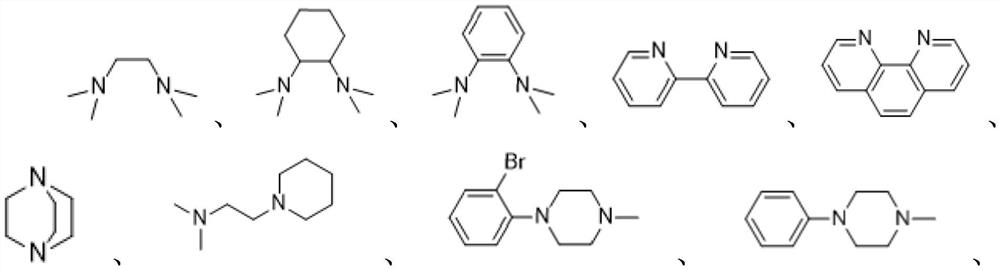
[0026] L is a diamine ligand coordinated with metal Fe, selected from the following compounds:
[0027]
[0028]
[0029] According to the present invention, in the existing method for preparing cyclic ethylene (propylene) carbonate, most of them exist when the conversion rate of alkylene oxide reaches a certain level, and the reaction rate drops sharply due to the decrease of reactant concentration, resulting in the need for more It takes a long time to realize the problem of relatively complete conversion of alk...
preparation example 1
[0072] The preparation of preparation example 1 complex catalyst
[0073] Add 800mL of ethanol as a solvent in a 2L reactor to dissolve 6.48g of FeCl 3 (0.04mol) and 4.64g ligand (0.04mol), slowly dropwise added a mixed solution of 10.4g KCN (0.16mol) and 500mL ethanol into the reactor, and stirred at reflux at 78°C for 6h after the dropwise addition. After the reaction is finished, cool and suction filter, move the filtrate to a stirred reactor, slowly add 49g ZnCl 2 (0.36mol), stirred at 50°C for 4h, cooled and filtered after the reaction was completed, and vacuum-dried the obtained solid to obtain 52g of the target product. The structural formula of the obtained product is (ZnCl)[Fe(CN) 4 ·L]·(ZnCl 2 ) 7~8 (the value of n is calculated according to the molar weight), L is The following percentages were determined by elemental analysis: Zn=39.3%, Fe=3.9%, consistent with theory.
Embodiment 1
[0075] 200 Kg of ethylene carbonate containing 80 g of the complex catalyst of Preparation Example 1 was added to a circulating loop jet flow reactor with an effective volume of 1000 L. The reaction device was started, and the starting material was heated to 150° C. through a heat exchanger, and carbon dioxide was introduced until the pressure of the reaction system was 3 MPa. Continuously add 400Kg ethylene oxide and 400Kg carbon dioxide (equimolar amount) within 5 hours, need to keep the feeding amount of alkylene oxide and carbon dioxide consistent during the feeding process, and system pressure maintains at 3MPa. After the addition was finished, the reaction was continued for 30 minutes. The reaction material is transferred to the flash tank, and after the carbon dioxide is discharged, about 800Kg of ethylene carbonate (selectivity>99.5%) is obtained by vacuum distillation, and the residual liquid containing the catalyst is recycled as the starting material.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap