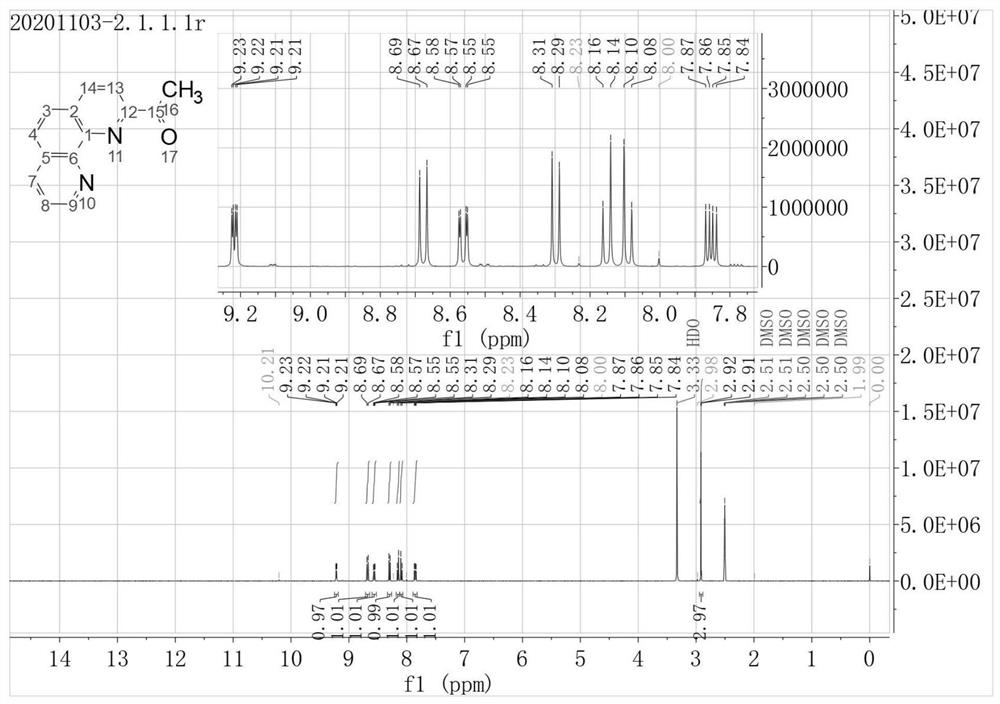
Preparation method of 2-acetyl-1, 10-phenanthroline
A technology of phenanthroline and acetyl, which is applied in the field of preparation of 2-acetyl-1,10-phenanthroline, can solve the problems of high storage conditions, unrealized large-scale industrial production, and difficult control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention provides a kind of preparation method of 2-acetyl-1,10-phenanthroline, comprising the following steps:
[0024] Mix 8-aminoquinoline, aceguvaldehyde and alcohol organic solvents, and undergo a Schiff base reaction to obtain E-1-(quinoline-8-imino)propane-2-one;
[0025] Mix the E-1-(quinoline-8-imino)propane-2-one with absolute ethanol, add catalyst and pyruvic acid after removing water, and doebner reaction occurs to obtain 2-acetylphenanthroline -4-Formic acid;
[0026] The 2-acetylphenanthroline-4-carboxylic acid undergoes a decarboxylation reaction under alkaline conditions to obtain the 2-acetylphenanthroline-1,10-phenanthroline.
[0027] In the present invention, unless otherwise specified, all raw material components are commercially available products well known to those skilled in the art.
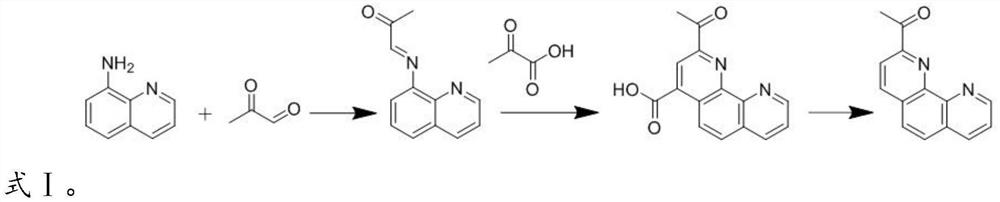
[0028] In the present invention, the preparation process of the 2-acetyl-1,10-phenanthroline is preferably as shown in formula I:
[0029]
[0030] The ...
Embodiment 1
[0057] At room temperature, dissolve 36g (0.25mol) of 8-aminoquinoline in 180mL of ethanol, add dropwise 20g (0.28mol) of methylglyoxal, stir for 3min, react at 55°C for 2h, cool down to -5°C, and keep warm 1h, filter, the obtained filter cake is rinsed with cold ethanol, sucked dry, and vacuum-dried at 55°C for 10h to obtain 50.2g E-1-(quinoline-8-imino)propane-2-one ( Yield 95%, purity 98%);
[0058] After mixing 50g (0.25mol) of E-1-(quinoline-8-imino)propan-2-one and 250mL of anhydrous methanol, reflux for 3h, then add 23g of PPA and 0.5g of vanadic acid, dropwise add 23g (0.26mol) pyruvic acid, heated to reflux for 12h, recovered methanol, added 250mL of purified water and 150mL of dichloromethane, stirred at room temperature for 2h, stood to separate layers, took the organic layer and washed it with water until pH = 7, to obtain 2-acetylphenanthrolephrine Phenyl-4-carboxylic acid;
[0059] The 2-acetylphenanthroline-4-formic acid was adjusted to pH 10.5 with 10% sodium...
Embodiment 2
[0062] At room temperature, dissolve 36g (0.25mol) of 8-aminoquinoline in 180mL of ethanol, dropwise add 20g (0.28mol) of methylglyoxal, stir for 3min, react at 70°C for 2h, cool down to -5°C, and keep warm 1h, filtered, rinsed the obtained filter cake with cold ethanol, sucked dry, and dried in vacuum at 55°C for 10h to obtain 49g of E-1-(quinoline-8-imino)propan-2-one;
[0063] Mix 40g (0.20mol) of E-1-(quinoline-8-imino)propan-2-one and 220mL of anhydrous methanol, reflux for 3h, then add 35g of phosphorus oxychloride, dropwise add 21g (0.24mol ) pyruvic acid, heated to reflux for 15h, reclaimed methanol, added to a mixture of 200mL purified water and 150mL dichloromethane, stirred at room temperature for 2h, stood to separate layers, took the organic layer and washed it with water until pH = 7, to obtain 2-acetyl Phenanthroline-4-carboxylic acid;
[0064] The 2-acetylphenanthroline-4-formic acid is adjusted to pH 10.5 with a mass concentration of 10% sodium hydroxide solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com