Method for separating nickel and iron from nickel-iron alloy and application
A technology of nickel-iron alloy and nickel-iron, which is applied in the direction of iron oxide/iron hydroxide, nickel sulfate, process efficiency improvement, etc., can solve the problems of high impurity content, high cost, poor quality of iron phosphate, etc., and achieve simple process flow, Great achievability, low acid consumption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
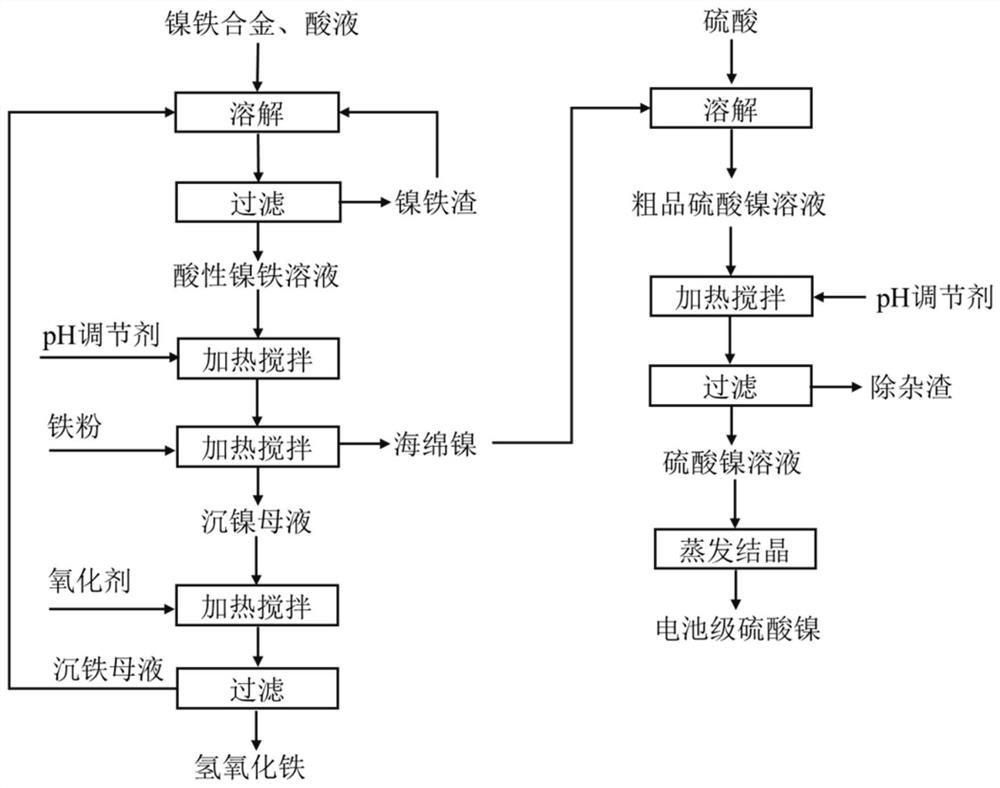
[0042] The method for separating nickel and iron from nickel-iron alloy of the present embodiment may further comprise the steps:
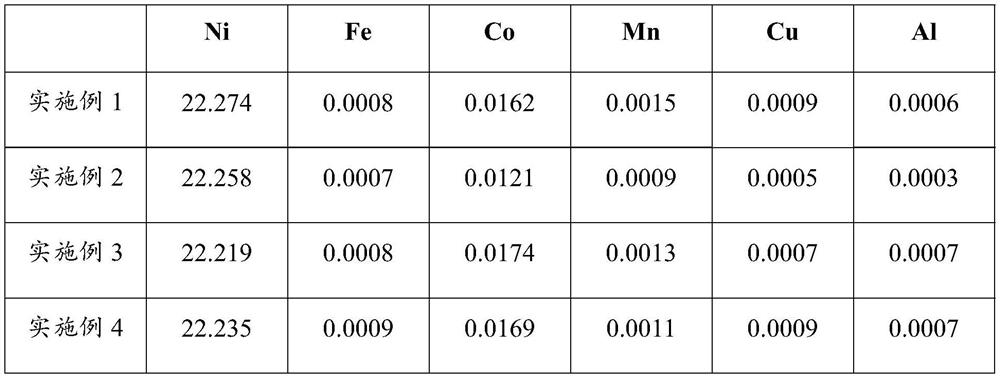
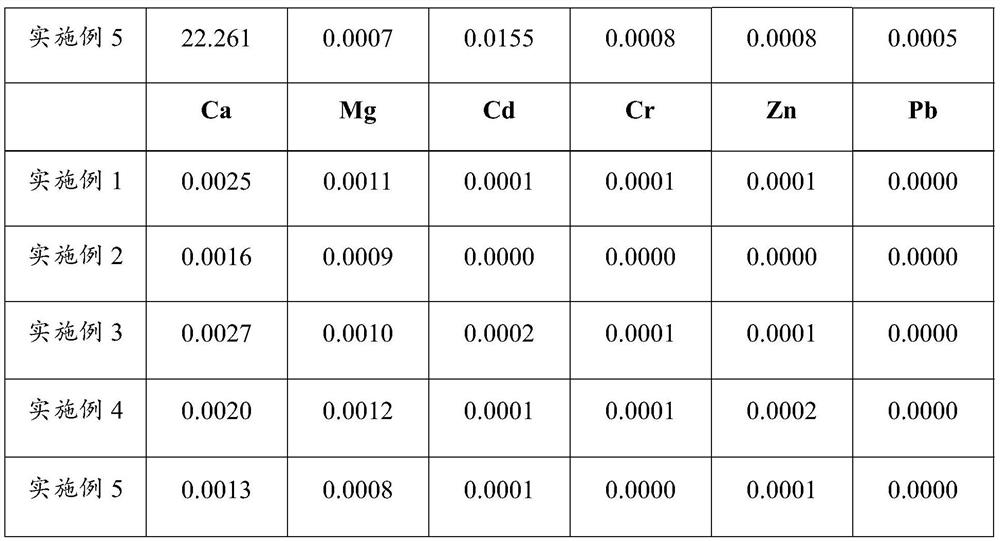
[0043] (1) Weigh 200g of nickel-iron alloy (iron: 63.28%, nickel: 35.51%, Co: 0.34%, Mn: 0.09%, Si: 0.27%, Cr: 0.05%, Ca: 0.006%, Mg: 0.004%, Cu :0.03%, S:0.21%, P:0.03%), add 2.5mol / L sulfuric acid solution for 1:8g / mL by solid-liquid ratio, dissolve at 80 ℃, after filtering, collect filtrate, obtain acid ferronickel Leachate;
[0044] (2) Add nickel-iron alloy powder to the ferronickel leaching solution obtained in step (1) to adjust the pH of the solution to 1.5, the time for adjusting the pH is 2h, and add iron powder of 0.8 times the theoretical amount required to replace nickel at 70°C After the replacement, filter to obtain sponge nickel and nickel sink mother liquor;
[0045] (3) adding hydrogen peroxide to the heavy nickel mother liquor obtained in step (2), ferrous iron in the solution can be oxidized to ferric iron and then hydrolyzed...
Embodiment 2
[0051] The method for separating nickel and iron from nickel-iron alloy of the present embodiment may further comprise the steps:
[0052] (1) Weigh 150g of nickel-iron alloy (iron: 83.12%, nickel: 15.45%, Co: 0.51%, Mn: 0.05%, Si: 0.36%, Cr: 0.09%, Ca: 0.012%, Mg: 0.008%, Cu : 0.05%, S: 0.22%, P: 0.01%) is 1: 6g / mL adding 2.5mol / L sulfuric acid solution according to mass solid-liquid ratio, dissolves at 85 ℃, filters, and gets filtrate to obtain acid ferronickel leaching solution;
[0053] (2) Add sodium carbonate to the acid nickel-iron leaching solution obtained in step (1) to adjust the pH of the solution to 1.8, the time for pH adjustment is 3h, and add 0.9 times the theoretical amount of iron required to replace nickel at 60°C Powder, after the replacement, filter to obtain sponge nickel and nickel sink mother liquor;
[0054] (3) The nickel-precipitated mother liquor obtained in step (2) is fed into oxygen, ferrous iron in the solution can be oxidized to ferric iron an...
Embodiment 3
[0059] The method for separating nickel and iron from nickel-iron alloy of the present embodiment may further comprise the steps:
[0060] (1) Weigh 100g of nickel-iron alloy (iron 67.22%, nickel 30.79%, Co: 0.47%, Mn: 0.08%, Si: 0.25%, Cr: 0.09%, Ca: 0.008%, Mg: 0.005%, Cu: 0.04 %, S: 0.29%, P: 0.05%), add 2mol / L hydrochloric acid solution according to the solid-liquid ratio of 1:8g / mL, dissolve at 65°C, filter, and take the filtrate to obtain the acid nickel-iron leaching solution;
[0061] (2) Add ammonia water to the acidic nickel-iron leaching solution obtained in step (1) to adjust the pH of the solution to 2.0, adjust the pH time for 4 hours, and add iron powder at 65°C that is 0.85 times the theoretical amount required to replace nickel. Filtration after the end to obtain sponge nickel and nickel sink mother liquor;
[0062] (3) Pass the heavy nickel mother liquor obtained in step (2) into air, ferrous iron in the solution can be oxidized to ferric iron and then hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com