Mhs cell-derived exosome-loaded forsythin drug delivery system and its application
A drug delivery system and exosome technology, applied in the field of biomedicine, can solve the problems of poor absorption and low bioavailability, and achieve the effect of prolonging the half-life, good anti-tumor metastasis effect, and good anti-tumor cell metastasis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
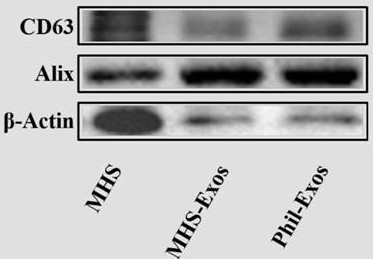
[0021] Example 1. Preparation of MHS cell-derived exosomes loaded with forsythin drug delivery system (Phil-Exos)
[0022] 1. Reagents and cells
[0023] Fetal bovine serum (FBS), American Gibco Company; RPMI 1640 medium, Hyclon Company; Forsythin reference substance, batch number MUST-14052103, mass fraction ≥ 98%, Chengdu Institute of Biology, Chinese Academy of Sciences; pre-stained protein Marker (10- 180kDa), American Thermo Company; SDS-PAGE gel preparation kit, blocking solution BSA, Kangwei Century Company; CD63, Alix, β-Actin (internal reference) antibody, HRP-labeled IgG, Wuhan Sanying Biotechnology Co., Ltd.; PBS (pH=7.4), penicillin-streptomycin (double antibody, 100IU·mL -1 ), RIPA cell lysate, BCA protein quantification kit, ECL Plus ultra-sensitive luminescence solution, Beijing Solarbio Technology Co., Ltd.; PKH67 staining kit, Beyotime; DAPI staining solution, DiI staining kit, Beyotime Company; 4% poly Formaldehyde general-purpose tissue fixative, biosharp ...
Embodiment 2
[0030] Particle size and Zeta potential measurement of embodiment 2, MHS-Exos and Phil-Exos
[0031] Take 100 μL of MHS-Exos and Phil-Exos suspensions respectively, dilute them to 1 mL with ultrapure water, and equilibrate at room temperature for 2 minutes, then measure their particle size and Zeta potential with a Malvern nanoanalyzer, and repeat 3 times.
[0032] see results figure 1 , the average particle size of MHS-Exos is (126.78±1.98)nm, the dispersion coefficient of PDI is 0.332±0.061, the Zeta potential is (-18.25±0.05)mV; the average particle size of Phil-Exos is (138.93±0.98)nm, the PDI The dispersion coefficient is 0.237±0.023; the Zeta potential is (-19.33±0.17)mV. The particle size results showed that exosomes had a narrow particle size distribution range, the particle size increased slightly after drug loading, and the potential did not change significantly before and after drug loading. The operation of drug loading had little effect on the surface protein con...
Embodiment 3
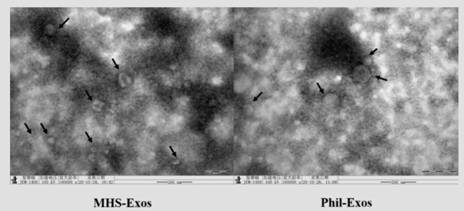
[0033] Embodiment 3, transmission electron microscope observation form
[0034] Take 10 μL of the MHS-Exos and Phil-Exos suspension and drop it on the copper grid, let it stand for 1 min, then blot the remaining liquid with filter paper, then add 3 drops of 3% phosphotungstic acid solution for negative staining at room temperature for 5 min, and blot the remaining liquid with the filter paper. Dry at room temperature and observe under a transmission electron microscope, and take pictures to record the shape of exosomes.
[0035] see results figure 2 , the diameter of exosomes slightly increased after loading Phil, which was consistent with the results of Malvern particle size. Both MHS-Exos and Phil-Exos showed a round or oval shape with a complete membrane structure, ranging in size, with a diameter of about 30-150nm. The particles have good dispersibility, and the background is clean and clear.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com