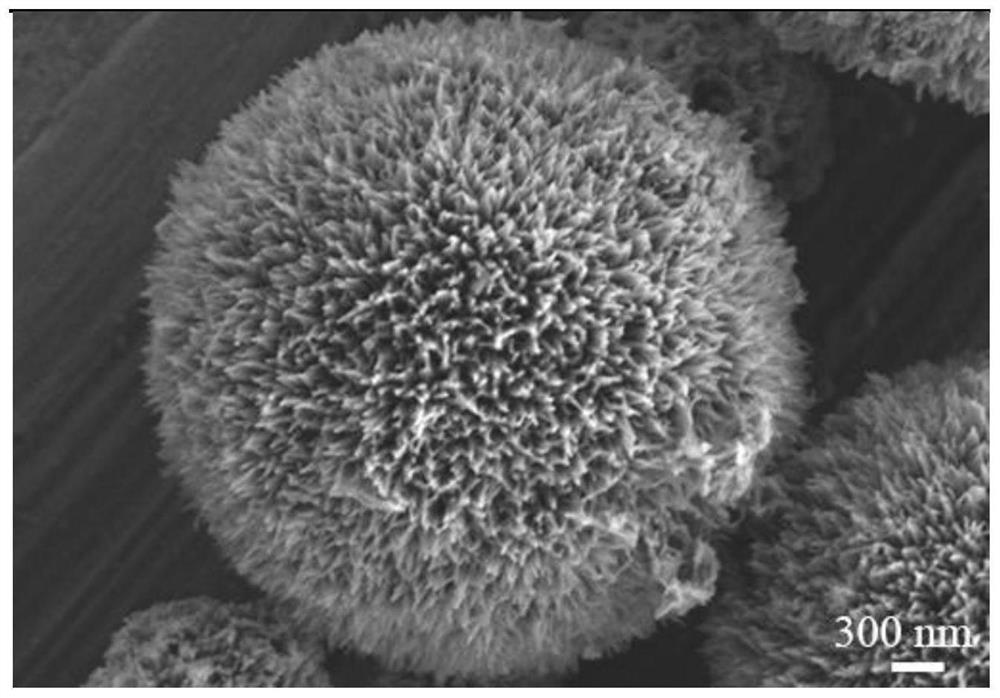
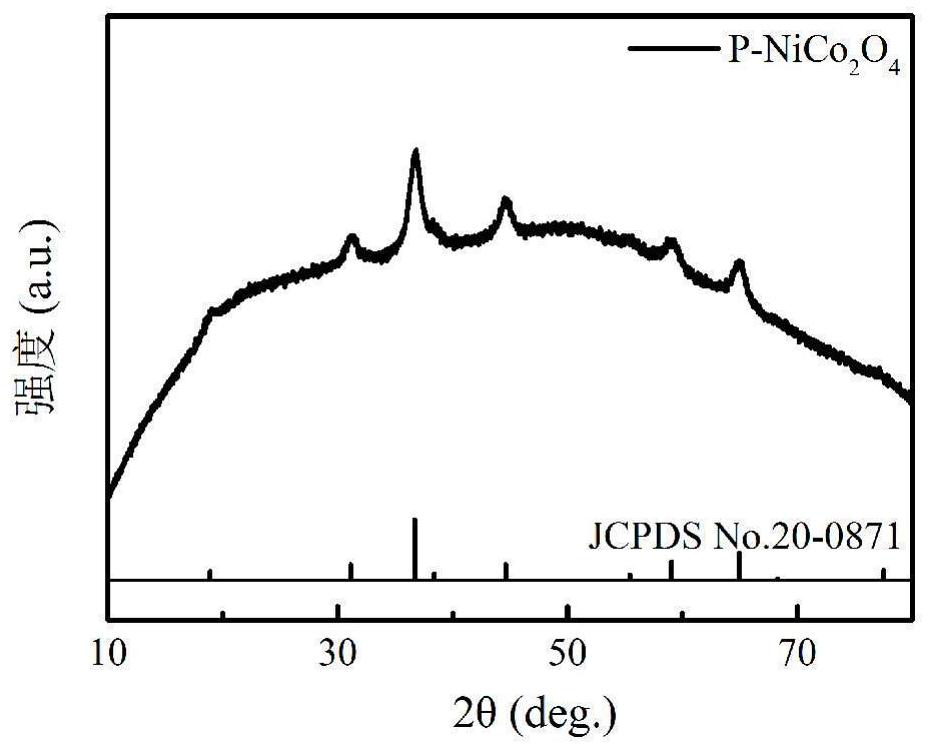
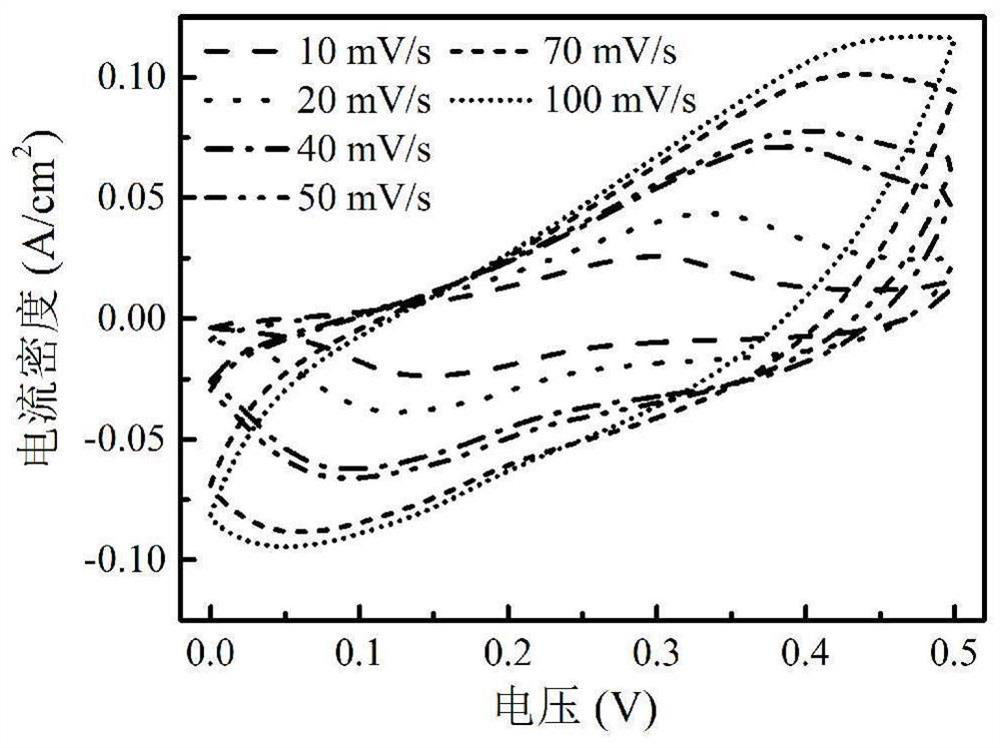
A flower-like phosphide nickel cobaltate material, preparation method and application
A phosphide cobalt acid, flower-like technology, applied in chemical instruments and methods, cobalt compounds, nickel compounds, etc., can solve the problems of poor stability of supercapacitors, limited fast electron transfer rates, and large changes in electrode volume expansion. Achieve the effect of enhancing pseudocapacitive capacity, improving volume expansion, and improving energy density and power density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of a flower-like phosphide nickel cobaltate material, comprising the following steps:
[0039] Step 1, prepare carbon nanosphere material:
[0040] (1.1) Weigh 20 g of glucose and dissolve it in 100 ml of deionized water, and stir for 10 h to obtain a glucose solution.
[0041] (1.2) Move the glucose solution obtained in step (1.1) to a polytetrafluoroethylene-lined hydrothermal reaction kettle for hydrothermal reaction at a temperature of 170° C. and a reaction time of 3 hours.
[0042] (1.3) The product after the hydrothermal reaction is subjected to solid-liquid separation using a centrifuge, and the resulting solid product is washed multiple times with deionized water and absolute ethanol, and the resulting solid product is placed in a drying box for drying to obtain carbon nanosphere material.
[0043] Step 2, preparation of flower-shaped nickel cobaltate material:
[0044] (2.1) At room temperature, 0.1g of carbon nanospheres, 1.16g of coba...
Embodiment 2
[0056] Step 1, prepare carbon nanosphere material:
[0057] (1.1) Weigh 30 g of glucose and dissolve it in 150 ml of deionized water, and stir for 12 hours to obtain a glucose solution.
[0058] (1.2) The glucose solution obtained in step (1.1) was transferred to a polytetrafluoroethylene-lined hydrothermal reaction kettle for hydrothermal reaction at a temperature of 190° C. and a reaction time of 5 hours.
[0059] (1.3) The product after the hydrothermal reaction is separated from the solid and liquid using a centrifuge, and the resulting solid product is washed multiple times with deionized water and absolute ethanol, and the resulting solid product is placed in a drying box for drying to obtain carbon nanosphere material.
[0060] Step 2, preparation of flower-shaped nickel cobaltate material:
[0061] (2.1) At room temperature, the 0.2g carbon nanospheres obtained in step 1, 2.32g cobalt nitrate hexahydrate (Co(NO 3 ) 2 ·6H 2 O), 1.16g nickel nitrate hexahydrate (Ni(...
Embodiment 3
[0069] Step 1, prepare carbon nanosphere material:
[0070] (1.1) Weigh 25 g of glucose and dissolve it in 150 ml of deionized water, and stir for 11 hours to obtain a glucose solution.
[0071] (1.2) Move the glucose solution obtained in step (1.1) to a polytetrafluoroethylene-lined hydrothermal reaction kettle for hydrothermal reaction at a temperature of 180° C. and a reaction time of 4 hours.
[0072] (1.3) The product after the hydrothermal reaction is separated from the solid and liquid using a centrifuge, and the resulting solid product is washed multiple times with deionized water and absolute ethanol, and the resulting solid product is placed in a drying box for drying to obtain carbon nanosphere material.
[0073] Step 2, preparation of flower-shaped nickel cobaltate material:
[0074] (2.1) At room temperature, the 0.15g carbon nanospheres obtained in step 1, 2.4g cobalt nitrate hexahydrate (Co(NO 3 ) 2 ·6H 2 O), 1.2g nickel nitrate hexahydrate (Ni(NO 3 ) 2 ·...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| retention rate | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com