Preparation method of ambroxol hydrochloride impurity
A quality and salt-forming technology, which is applied in the field of preparation of ambroxol hydrochloride impurities, achieves the effects of high yield, real operation and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1, the synthesis of AXS-13-01
[0074]
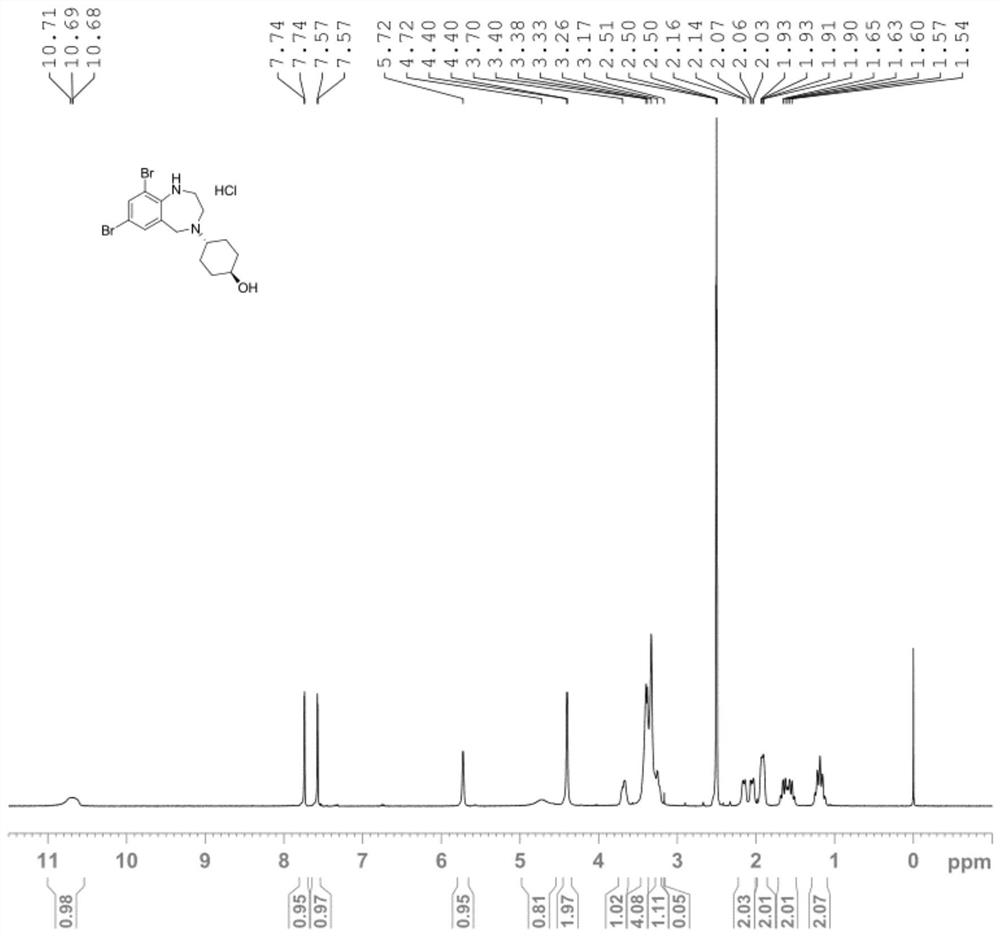
[0075] The specific steps are as follows: First, 10g (0.024mol) of ambroxol hydrochloride was dissolved in 100mL of dichloromethane solvent, 7.23g (0.072mol) of triethylamine was added, 0.29g (0.0024mol) of DMAP was stirred at room temperature for 30min, and then added 5.4g (0.036mol) TBSCl, be warming up to 40 ℃, stir 5 hours, TLC thin-layer chromatographic plate monitors (add DCM to dilute, developer: DCM:MeOH=20:1), treat that ambroxol hydrochloride raw material basically reacts, The reaction solution was lowered to room temperature, added water, stirred for 10 minutes, separated, the organic phase was washed with brine, dried and spin-dried, and purified by wet silica gel column chromatography to obtain the product, AXS-13-01, colorless oil, 10g, HPLC Purity: 99.20%, yield 85%.
Embodiment 2
[0076] Embodiment 2, the synthesis of AXS-13-02
[0077]
[0078] The specific steps are as follows: First, add 10g (0.02mol) AXS-13-01 to 50mL acetonitrile, add 5.26g (0.041mol) DIEA, 1.69g (0.010mol) KI, then dropwise add 4.4g (0.022mol) bromine Tert-butyl acetate, dropwise, warming up to reflux and stirring for 8 hours, TLC thin-layer chromatographic plate monitoring (adding DCM for dilution, developer: PE:EA=1:1) reaction, when the AXS-13-01 raw material completely disappeared The reaction solution was lowered to room temperature, added water and EA, stirred for 10 min, separated, the organic phase was washed with brine, dried and spin-dried, wet silica gel column chromatography, PE: EA = 50: 1, washed out the product, enriched and concentrated to obtain AXS -13-02, yellow oil, 9g, HPLC purity: 98.82%, yield 73%.
Embodiment 3
[0079] Embodiment 3, the synthesis of AXS-13-03
[0080]
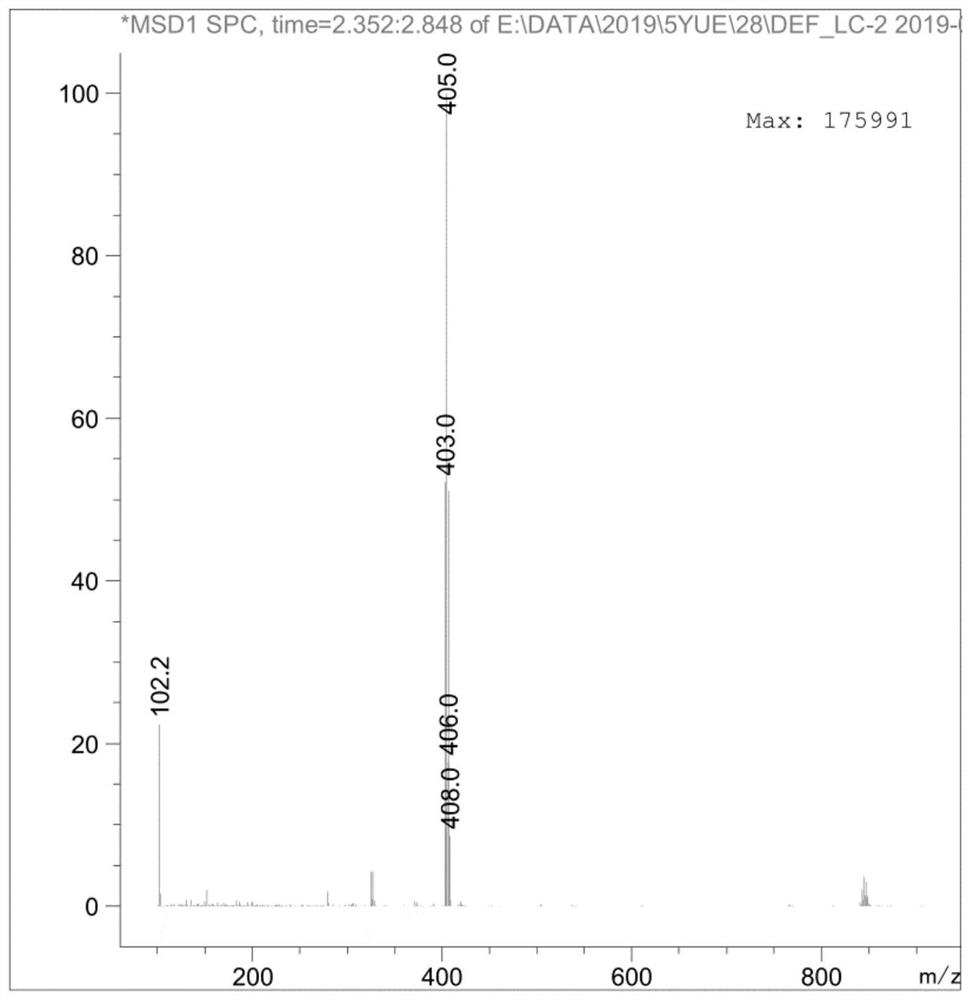
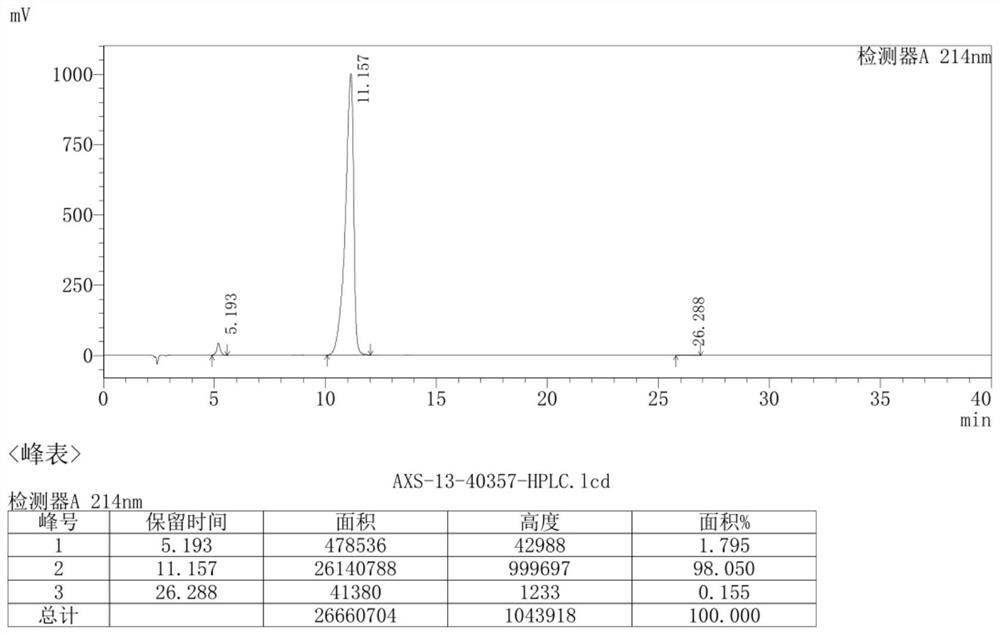
[0081] The specific steps are as follows: 9g (0.015mol) AXS-13-02 was added to 90mL THF, 2.5g (0.023mol) t-BuOK was added in batches, stirred at room temperature for 2 hours, and monitored by TLC thin layer chromatography (adding water and EA , take the EA phase, developing agent: PE:EA=2:1), the reaction of the raw material AXS-13-02 is completed. Add saturated ammonium chloride and EA, stir for 10min, separate the layers, wash the organic phase with brine, dry and spin dry to obtain AXS-13-03, yellow solid, 7.64g, HPLC purity: 96.37%, yield 96%, its mass spectrum is as follows: Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com