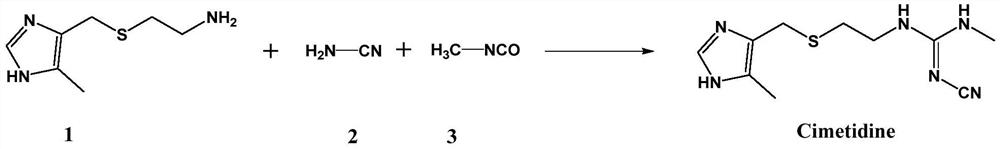
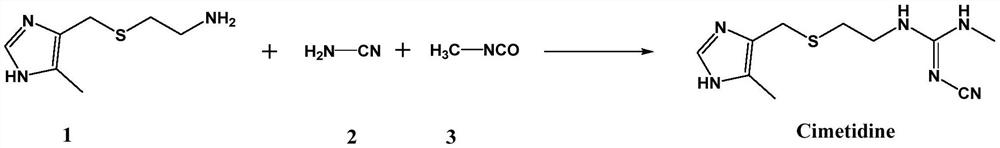
Process for synthesizing cimetidine
A cimetidine and process technology, applied in the field of synthesizing cimetidine, can solve the problems of not overcoming the reaction route, having malodorous methyl mercaptan, long reaction route and the like, avoiding the malodor problem, improving environmental protection and safety , the effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 4-((2-aminoethyl)thiomethyl)-5-methylimidazole (2.06g, 12.0mmol), cyanamide (0.53g, 12.6mmol), methyl isocyanate in a 250mL three-necked flask Ester (0.72g, 12.6mmol), CuI (0.11g, 0.6mmol), triphenylphosphine (0.31g, 1.2mmol), toluene (100mL), stirred for 10min, then heated to reflux to continue the reaction. The reaction progress was monitored by TLC until the point where the raw material 4-((2-aminoethyl)thiomethyl)-5-methylimidazole completely disappeared, and then the reaction was continued for 2 hours, and the total reaction time was about 8 hours. After the reaction, the reaction solution was naturally cooled to room temperature, washed with 0.1M aqueous hydrochloric acid (100 mL), and then washed with saturated sodium chloride solution (100 mL) and water (100 mL×2). The oil phase was dried with anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was separated by column chromatography. The eluent was ethyl acetate / pe...
Embodiment 2
[0024] Add 4-((2-aminoethyl)thiomethyl)-5-methylimidazole (2.06g, 12.0mmol), cyanamide (0.53g, 12.6mmol), methyl isocyanate in a 250mL three-necked flask Ester (0.72g, 12.6mmol), Cu2O (0.086g, 0.6mmol), triphenylphosphine (0.31g, 1.2mmol), toluene (100mL), stirred for 10min, then heated to reflux to continue the reaction. The reaction progress was monitored by TLC until the point where the raw material 4-((2-aminoethyl)thiomethyl)-5-methylimidazole completely disappeared, and then the reaction was continued for 2 h, and the total reaction time was about 10 h. After the reaction, the reaction solution was naturally cooled to room temperature, washed with 0.1M aqueous hydrochloric acid (100 mL), and then washed with saturated sodium chloride solution (100 mL) and water (100 mL×2). The oil phase was dried with anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was separated by column chromatography, and the eluent was ethyl acetate / pet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com