Antigen composition for detecting mycoplasma bovis antibody, kit and application thereof
A technology of Mycoplasma bovis and a composition is applied in the field of animal infectious disease prevention and treatment, which can solve the problems of cross-agglutination, insufficient sensitivity, misjudgment of clinical serum antibody diagnosis, etc., and achieves the effect of high detection rate, balancing reactogenicity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
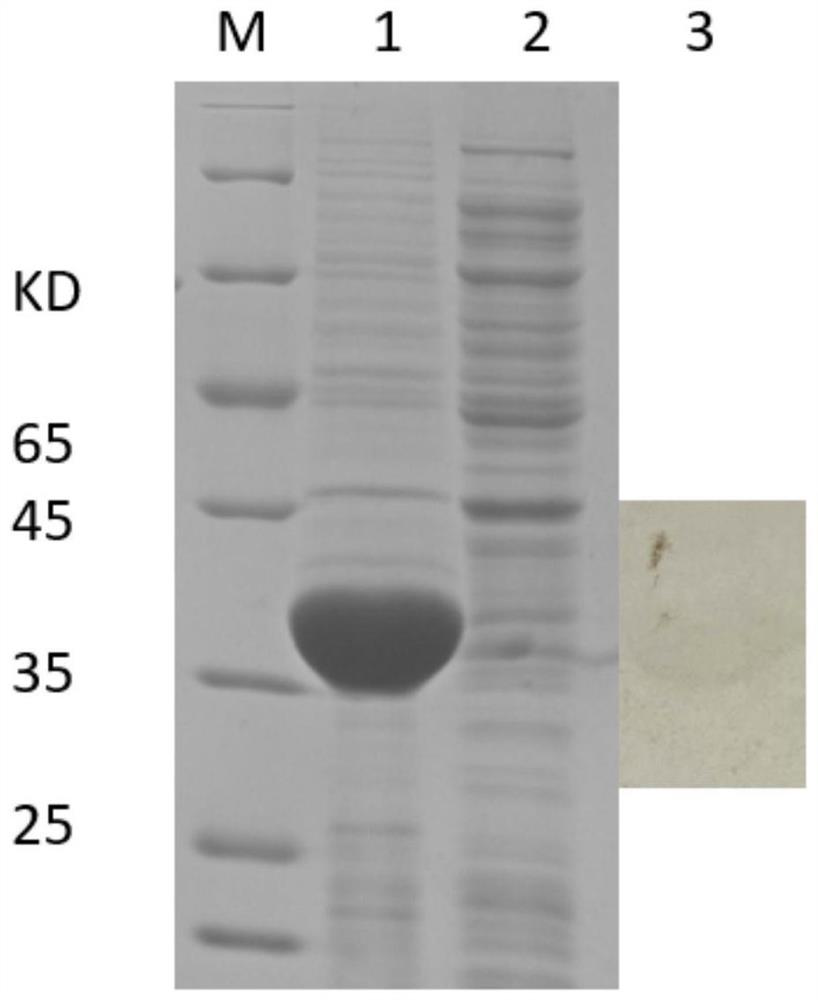
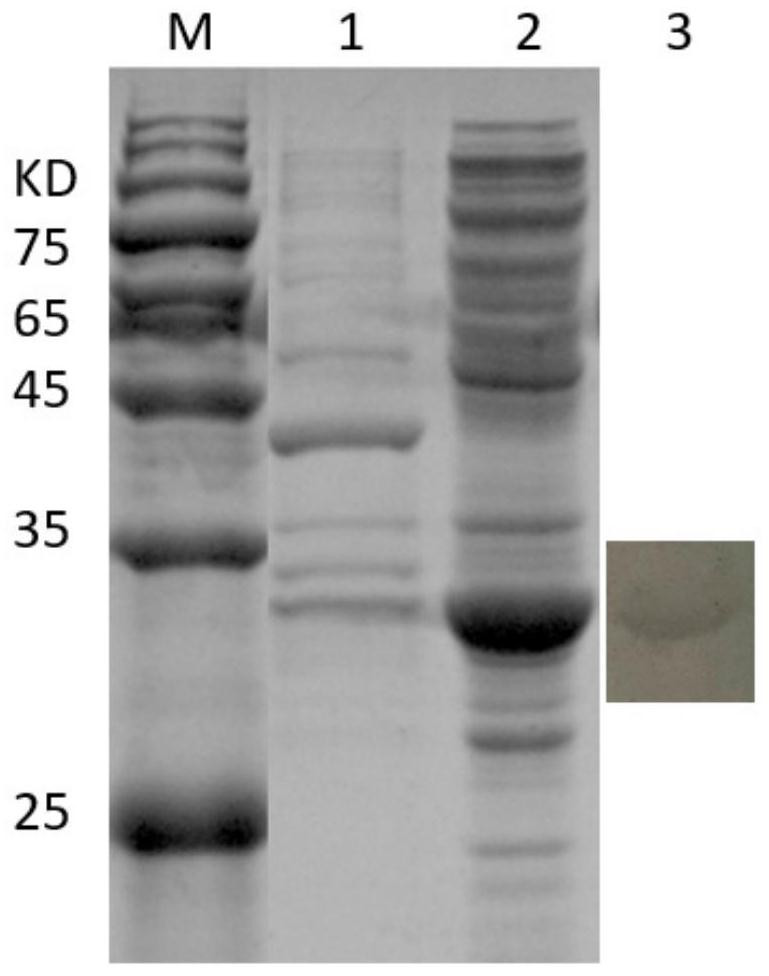
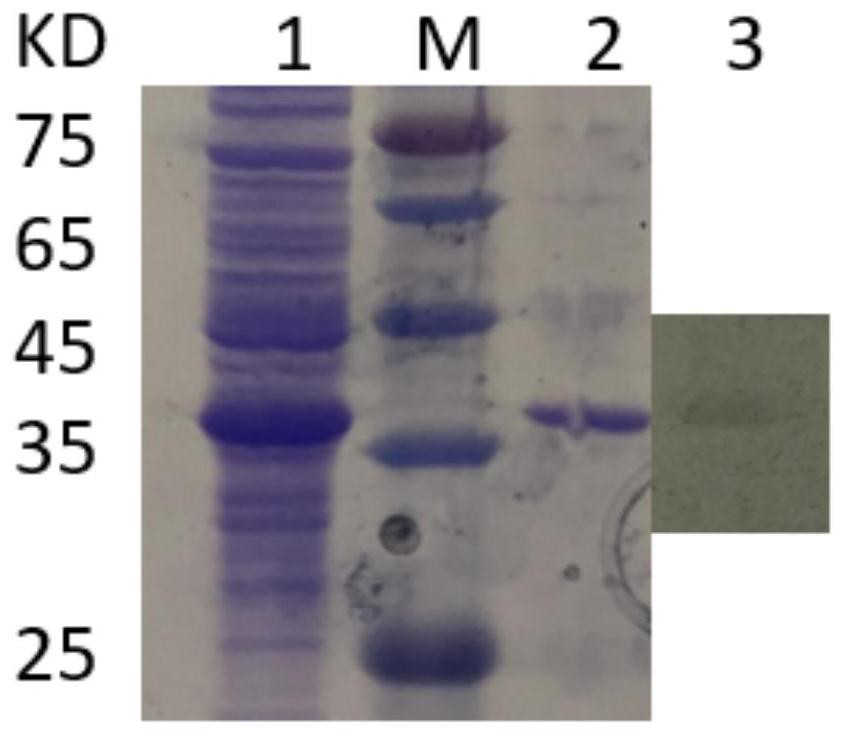
[0034] Embodiment 1, the acquisition of mycoplasma bovis specific antigenic protein
[0035] 1.1 Acquisition of complete gene sequences of different strains of Mycoplasma bovis
[0036] The full gene sequences of 33 representative strains of Mycoplasma bovis (Mycoplasma bovis) were found from the National Center for Biotechnology Information (NCBI) in the United States. , Journal of Livestock Ecology, 2017, 38(2):64-66" 1.4, hereinafter referred to as "Mycoplasma bovis Shandong strain"), and its full gene sequence was obtained through company sequencing.
[0037] 1.2 Computer prediction analysis of antigenic protein
[0038] Using online biological software PSORTb, Vaxign 2Beta, BLASTp, etc. to analyze the protein localization, transmembrane region, antigen parameters and homology comparison of different M. bovis strains, the following candidate M. bovis specific antigen proteins 4979.1, 0148.1, 2700.1 were obtained.
[0039] 1.3 Codon optimization of candidate Mycoplasma bo...
Embodiment 2
[0056] Embodiment 2, the reactogenicity analysis of different Mycoplasma bovis specific antigenic protein
[0057] The purified antigenic protein obtained in step 1.4 of Example 1 was respectively coated on the reaction wells of the ELISA plate according to the coating concentration gradient of 32.0, 16.0, 8.0, 4.0, 2.0, 1.0, 0.5, 0.25 μg / mL.
[0058] The inactivated culture of Mycoplasma bovis Shandong strain was used to immunize New Zealand rabbits to prepare positive serum for the study of its reactogenicity, and the serum of non-immunized New Zealand white rabbits was used as negative control.
[0059] Preparation of buffer:
[0060] Coating buffer (pH9.6 carbonate buffer): 1.59g of sodium carbonate, 2.93g of sodium bicarbonate, dilute to 1000ml with ultrapure water, confirm that the pH value is 9.6 with a pH meter, filter with a 0.44μm membrane Backup.
[0061]Washing buffer: weigh 8.0g sodium chloride, 0.2g potassium dihydrogen phosphate, 6.29g disodium hydrogen phosph...
Embodiment 3
[0087] Embodiment 3, the reaction specificity analysis of Mycoplasma bovis specific antigenic protein
[0088] Carry out according to the method of embodiment 2, difference is: 4979.1, 0148.1 and 2700.1 proteins use 2μg / ml, 8μg / ml and 20μg / ml respectively as coating antigen to carry out the coating of ELISA plate; The source positive serum was replaced with positive serum of Mycoplasma agalactiae, Mycoplasma alcaligenes, Mycoplasma mycoplasma subsp. goat, or Mycoplasma subsp. filamentous for specificity determination.
[0089] The preparation method of the positive serum of above-mentioned Mycoplasma agalactiae, Mycoplasma alcaligenes, Mycoplasma filamentous subspecies goat subspecies, or Mycoplasma filamentous subspecies is as follows:
[0090] Mycoplasma agalactiae (CVCC344), Mycoplasma alcaligenes (YX), Mycoplasma filiformis subspecies goat subspecies (CVCC3009), or Mycoplasma filamentous subspecies filamentous subspecies (CVCC378) were all from Binzhou animal husbandry Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com