Benzimidazole anion exchange membrane and preparation method thereof
An anion-exchange membrane, benzimidazole technology, applied in the field of benzimidazole anion-exchange membrane and its preparation, can solve the problems of anion-exchange membrane IEC, low conductivity and water content, insufficient hydrophilicity, and reduce the ease of degradation of the main chain And other problems, to achieve the effect of high water content, high ion conductivity, excellent alkali resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 1,2,4,5-tetraaminobenzene
[0030] Weigh 1,2,3-trichlorobenzene (20.14g 0.11mol) into a three-neck flask, add 80mL of 98% concentrated sulfuric acid, heat to 50°C, start to add 20mL of concentrated nitric acid (65wt% ~ 68wt%) dropwise, and dropwise Afterwards, the temperature was raised to 70°C, the reaction was continued for 5 hours, and the reaction was stopped to obtain crude 4,6-dinitro-1,2,3-trichlorobenzene as a yellow solid with a yield of 82.4%;
[0031] Weigh 4,6-dinitro-1,2,3-trichlorobenzene (22.25g, 0.08mol) into a high-pressure reactor, add 50mL of ethylene glycol, heat to 150°C, and pass in ammonia gas, The ammonia pressure was 1.0 MPa, and the reaction was stopped after 8 hours of reaction. After cooling to room temperature, the reactant was filtered to obtain a crude product, which was recrystallized from absolute ethanol to obtain 4,6-dinitro-2-chloro-1,3-phenylenediamine. The yield was 86.5%.
[0032] Weigh 4,6-dinitro-2-chloro-1,3-phe...
Embodiment 2
[0034] Preparation of fluorinated polybenzimidazole (FPBI).
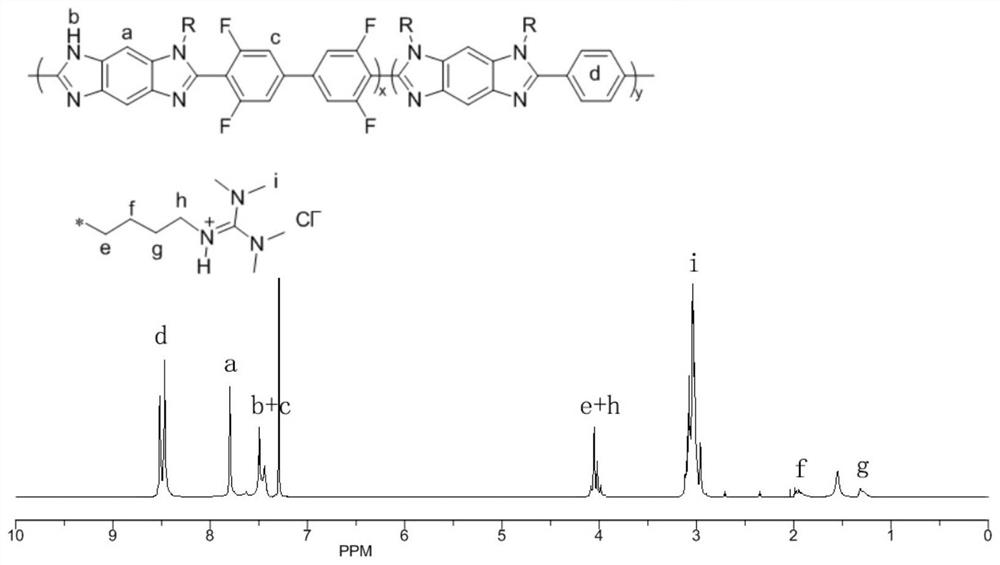
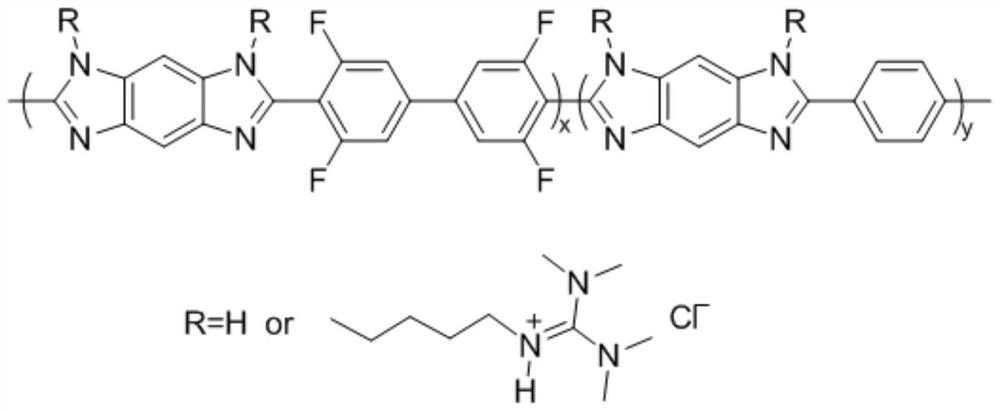
[0035] Set the program of the microwave synthesizer in advance according to the requirements, firstly in the N 2 In the presence of microwave radiation polyphosphoric acid (PPA) deoxidation 0.5h, mixed 1,2,4,5-tetraaminobenzene (TAB), terephthalic acid (PTA), 3,3',5,5'-tetra After fluorobiphenyl-4,4'-dicarboxylic acid (TFA) and PPA, the mixture was placed in N 2 Vigorously stirred under high temperature, using temperature programming method: 90°C for 0.5h, 120°C for 0.5h, 140°C for 5h, 180°C for 0.5h, 200°C for 10h. After the reaction is over, pour the reaction solution into an equal volume of deionized water to precipitate, filter and wash alternately with deionized water and ethanol, repeat several times until the water phase is neutral, and place the obtained dark solid in a vacuum oven at 100°C After drying for 48 hours, fluorine-containing polybenzimidazole (FPBI) was obtained.
[0036] The feeding quality, ...
Embodiment 3
[0042] Preparation of 1-bromobutyltetramethylguanidine hydrochloride (BGs).
[0043] Add 1,4-dibromobutane (29.02g, 0.13mol) in a reaction flask equipped with a constant pressure dropping funnel, add 80mL of ethanol and stir to dissolve, then start to drop tetramethylguanidine (5.16g, 0.04mol) , after the dropwise reaction was stirred at room temperature for 20 h; after the reaction, the reaction solution was filtered, and then the low boiling point compound was evaporated at 60° C. to obtain a crude product. Using dichloromethane as the eluent, it was passed through a silica gel column, and the resulting filtered fraction was dried to obtain a white solid powder, which was 1-bromobutyltetramethylguanidine, and the yield was 75.6%.
[0044] Dissolve 1-bromobutyltetramethylguanidine in 20mL of deionized water again, put it into a single-necked flask equipped with a constant pressure dropping funnel, raise the temperature to 60°C, start adding 20g of 30% hydrochloric acid soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com