RGA method for detecting biological activity of anti-CTLA-4 monoclonal antibody and application of RGA method
A CTLA-4, monoclonal antibody technology, applied in the field of biomedicine, can solve the problem of sensitivity to changes in the structure of anti-CTLA-4 monoclonal antibody drugs, and achieve the effects of short experimental period, simple operation, and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
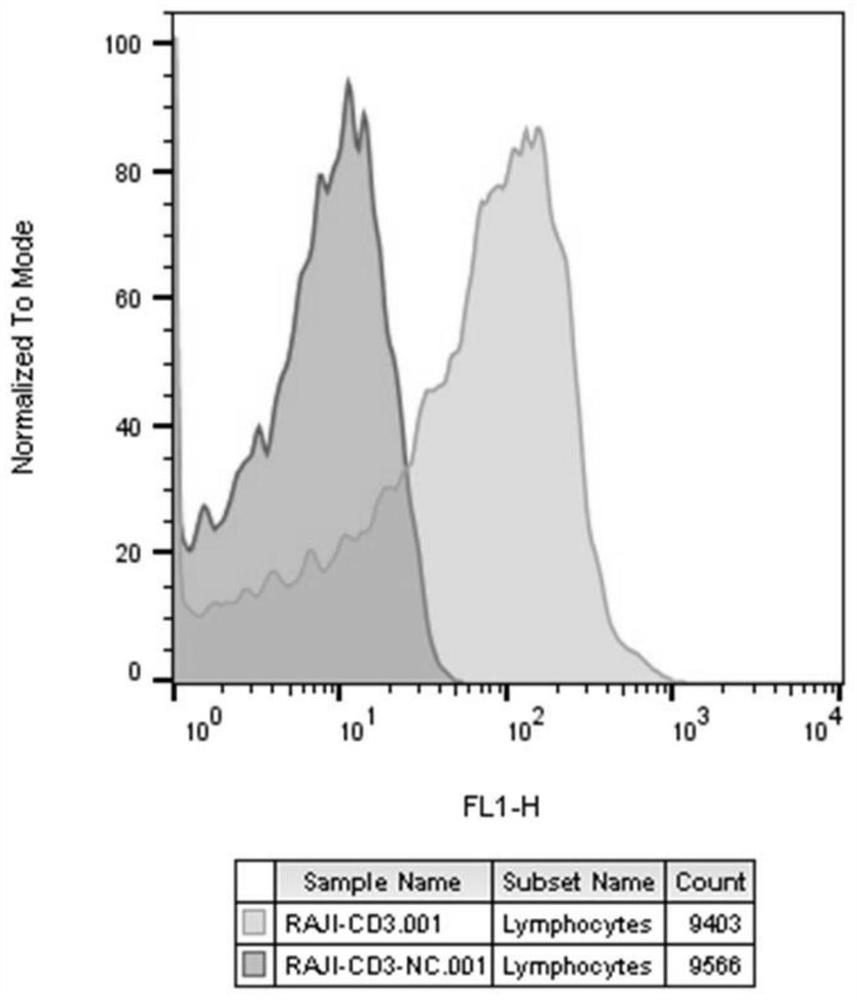
[0060] Example 1 Construction of Raji-CD3scFv cell line and Jurkat-CTLA-4-NFAT-luc cell line
[0061] 1. Construction of Raji cell line stably expressing CD3scFv
[0062] Using NEON TM Raji cells were transfected with CD3scFv plasmid using an electroporation transfection system (Invitrogen). 48h after transfection, Hygromycin B was added for pressure selection. After the cell density and viability were restored, five 96-well plates were plated at a rate of 0.5 cells / well. When the confluence of the cell clones in the wells reaches over 30%, a cell line stably expressing CD3scFv is obtained by flow cytometric screening, and is named Raji-CD3scFv cells.
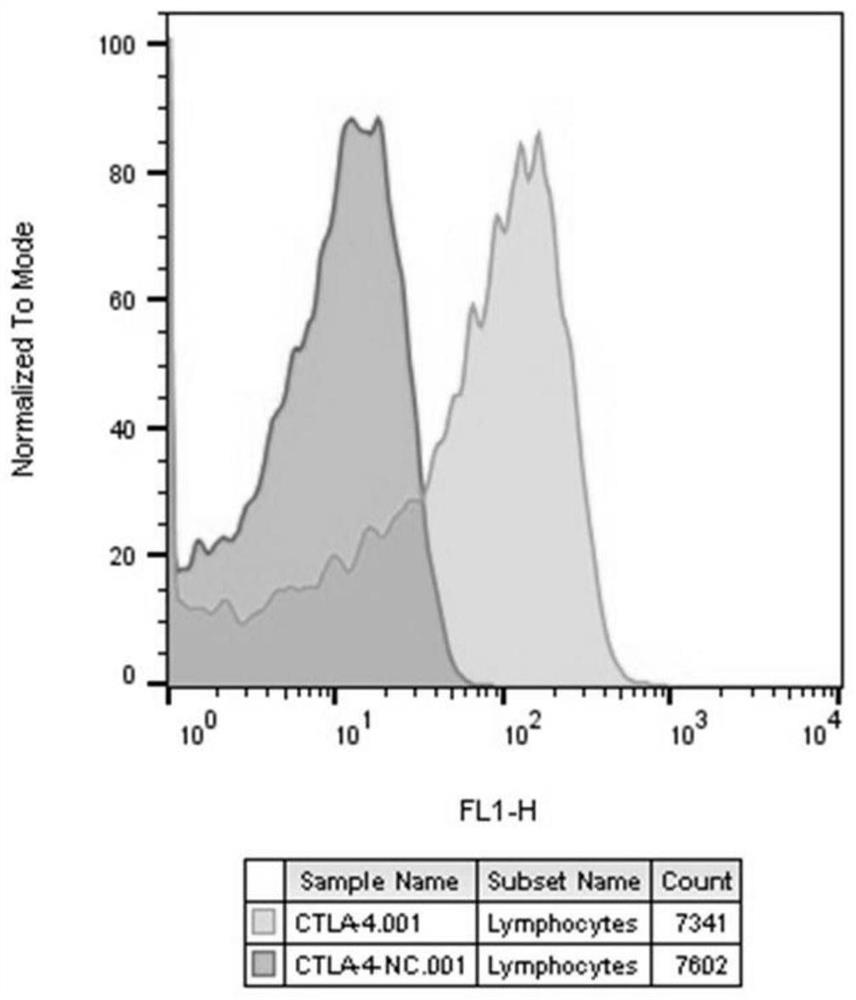
[0063] 2. Construction of Jurkat cell line stably expressing CTLA-4 gene and luciferase reporter gene expressed by NFAT response element
[0064] (1) Jurkat cells were stably transfected with luciferase reporter gene expressed by NFAT response element
[0065] Using NEON TM The electroporation transfection system (Invitro...
Embodiment 2
[0072] Example 2 Establishment of the RGA method for detecting the biological activity of anti-CTLA-4 monoclonal antibody
[0073] 1. Experimental method
[0074] (1) Sample preparation: Anti-CTLA-4 monoclonal antibody was diluted to 200 μg / mL with diluent, and then serially diluted according to the dilution ratio of 1:4, and 10 concentration gradients were set up, with 2-3 replicate wells for each concentration, 50 μL / Wells were added to a 96-well white plate.
[0075] (2) Preparation of Jurkat-CTLA-4-NFAT-luc effector cell suspension: collect effector cells in good growth state, centrifuge to discard supernatant, resuspend cells with diluent and count, adjust cell density to 6×10 6 pc / mL, spare.
[0076] (3) Preparation of Raji-CD3scFv target cell suspension: collect the target cells in good growth state, centrifuge to discard the supernatant, resuspend the cells with diluent and count them, and adjust the cell density to 6×10 5 pc / mL, spare.
[0077] (4) Cell plating: ...
Embodiment 3R
[0084] The optimization of embodiment 3RGA detection method
[0085] 1. Optimization of antibody dose-effect range
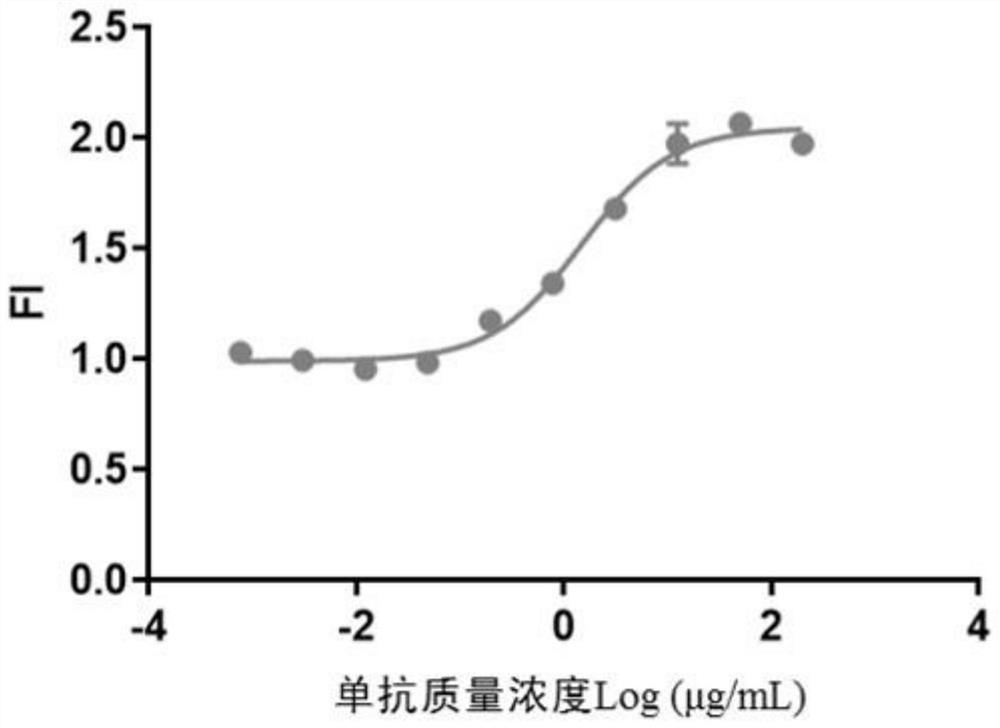
[0086] Use 200 μg / mL as the starting point for dilution of anti-CTLA-4 monoclonal antibody to conduct serial dilutions. The dilution ratios of the serial dilutions are set to 1:3, 1:4 and 1:5 respectively, and 10 concentration points are set. According to the measured chemiluminescence A four-parameter curve fitted to the values determined the range of action of anti-CTLA-4 mAbs.
[0087] Experimental method: Carry out experiment respectively according to the method described in embodiment 2, compare the dose-response curve of different dilution schemes at last, according to whether four parameter curves comprise upper and lower platform and points distributed on upper and lower platform and linear part Whether it is uniform to select the optimal dilution ratio.
[0088] Experimental results: see the experimental results Figure 4 , the results show that wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com