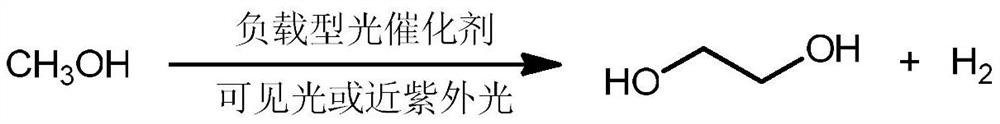
Method for preparing ethylene glycol through photocatalytic conversion of methanol
A technology of methanol conversion and ethylene glycol, which is applied in chemical instruments and methods, preparation of hydroxyl compounds, and preparation of organic compounds, etc., can solve problems that limit the practical application of ethylene glycol, and achieve energy friendliness, low environmental pollution, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] ZnSO 4 ·7H 2 O (1.0mmol, 287.6mg), InCl 3 4H 2 O (2.0mmol, 576.5mg) and NaCl (211.5mg) were added to an Erlenmeyer flask containing absolute ethanol, magnetically stirred at room temperature for 30min, and then thioacetamide (601.0mg) was added to the above mixture. After stirring for an additional 30 min, the mixture was transferred to a clean 50 mL Teflon-lined autoclave. After sealing, solvothermal reaction at 160°C for 20h. After the reaction, the autoclave was naturally cooled to room temperature. The reacted solid was separated by centrifugation and washed three times with absolute ethanol (25 mL), twice with ultrapure water (25 mL), and finally washed once with absolute ethanol. The obtained yellow solid was dried under vacuum at 60 °C for 12 h.
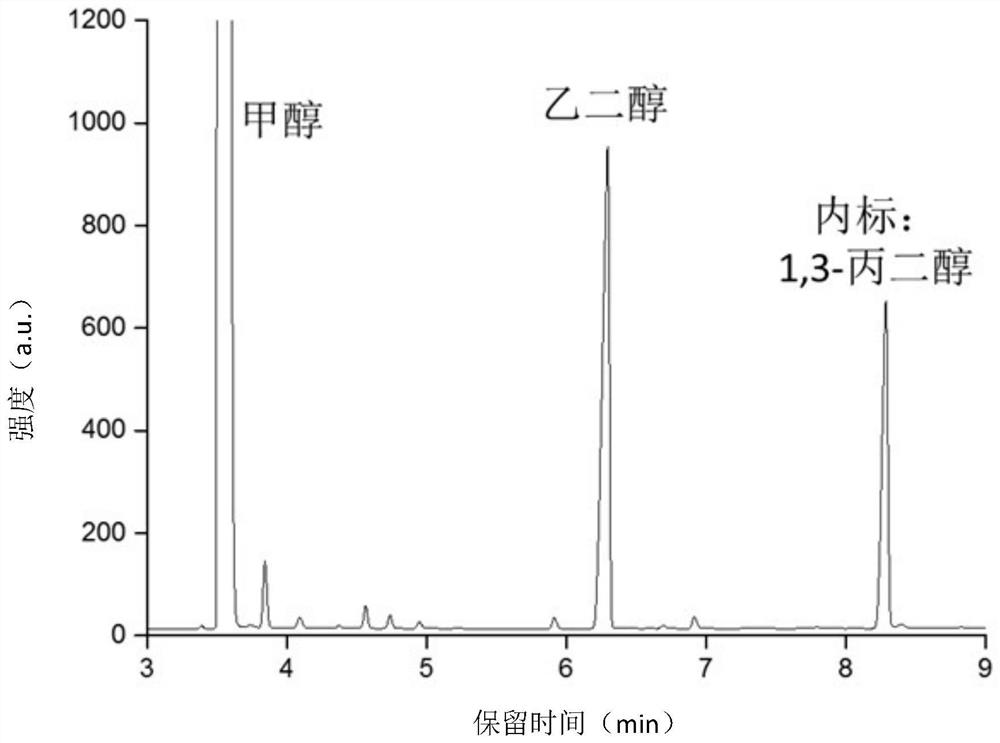
[0028] In a 220mL quartz pressure-resistant glass reactor, add 50mL of anhydrous methanol and 100mg of ZnIn 2 S 4 catalyst, and then replace the reactor with 5% volume fraction of CO / Ar and seal it. Under the l...
Embodiment 2
[0030] Solvothermal Preparation of ZnIn Using Isopropanol 2 S 4 / SiO 2 -40 catalyst. where 40 represents ZnIn 2 S 4 The mass of SiO 2 40% of the mass. SiO 2 (30 nm, hydrophilic) was dispersed in 30 mL of isopropanol and stirred for 2 h. ZnSO 4 ·7H 2 O (0.47mmol, 136.0mg), InCl 3 4H 2 O (0.95mmol, 277.2mg) and NaCl (100mg) were added to the above-mentioned isopropanol dispersion, and after magnetic stirring at room temperature for 30min, thioacetamide (284.2mg) was added. After stirring for an additional 30 min, the mixture was transferred to a clean 50 mL Teflon-lined autoclave. After sealing, solvothermal reaction was carried out in a rotary oven at 160° C. for 20 h. After the reaction, the autoclave was naturally cooled to room temperature. The reacted solid was separated by centrifugation and washed three times with absolute ethanol (25 mL), twice with ultrapure water (25 mL), and finally washed once with absolute ethanol. The obtained pale yellow solid was d...
Embodiment 3
[0033] Solvothermal Preparation of Zn Using Isopropanol 2 In 2 S 5 / SiO 2 -40 catalyst. where 40 represents Zn 2 In 2 S 5 The mass of SiO 2 40% of the mass. 500mg of SiO 2 (30 nm, hydrophilic) was dispersed in 30 mL of isopropanol and stirred for 2 h. ZnSO 4 ·7H 2 O (0.77mmol, 220.9mg), InCl 3 4H 2 O (0.77mmol, 225.2mg) and NaCl (100mg) were added to the above-mentioned isopropanol dispersion, and after magnetic stirring at room temperature for 30min, thioacetamide (288.6mg) was added. After stirring for an additional 30 min, the mixture was transferred to a clean 50 mL Teflon-lined autoclave. After sealing, solvothermal reaction was carried out in a rotary oven at 160° C. for 20 h. After the reaction, the autoclave was naturally cooled to room temperature. The reacted solid was separated by centrifugation and washed three times with absolute ethanol (25 mL), twice with ultrapure water (25 mL), and finally washed once with absolute ethanol. The obtained pale y...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron work function | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com