1, 3, 5, 7-tetramethylcyclotetrasiloxane-based ciprofloxacin fluorescent probe and application thereof in iron ion detection
A technology of tetramethylcyclotetrasiloxane ciprofloxacin and tetramethylcyclotetrasiloxane, which is applied in the field of fluorescence sensing, can solve problems such as interference and high detection limit, and achieve high application value and identification Effect of fast response, good selectivity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
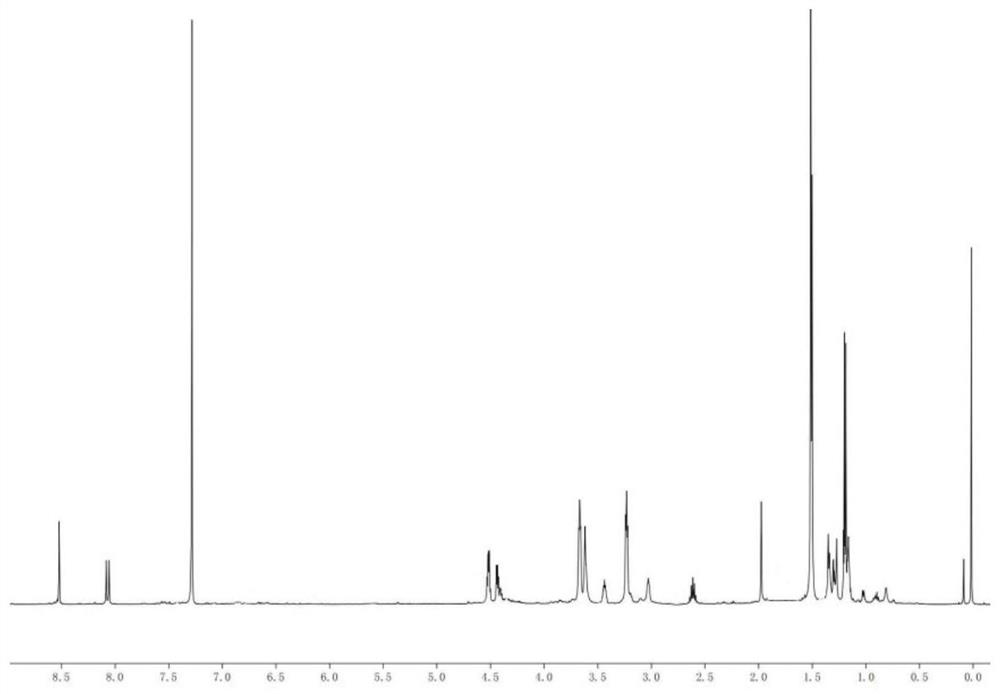
[0031] Dissolve 1.208mL (5.0mmol) of 1,3,5,7-tetramethylcyclotetrasiloxane in 2mL of anisole and place it in a 50mL two-neck flask, heat up to 80°C; add 120μL with a mass fraction of 1 % chloroplatinic acid-isopropanol solution; HBC 0.68g (1.25mmol) was dissolved in 20mL of anisole, and added dropwise to the two-neck flask; after reacting at 80°C for 8 hours; distillation under reduced pressure, the crude product Use chromatographic column separation and purification to obtain an orange-yellow solid, which is compound D 4 H-HBC.
Embodiment 2
[0033] Dissolve 0.604mL (2.5mmol) of 1,3,5,7-tetramethylcyclotetrasiloxane in 2mL of anisole and place it in a 50mL two-neck flask, heat up to 80°C; add 120μL with a mass fraction of 1 % chloroplatinic acid-isopropanol solution; HBC 0.68g (1.25mmol) was dissolved in 20mL of anisole, and added dropwise to the two-neck flask; after reacting at 80°C for 8 hours; distillation under reduced pressure, the crude product Use chromatographic column separation and purification to obtain an orange-yellow solid, which is compound D 4 H-HBC.
Embodiment 3
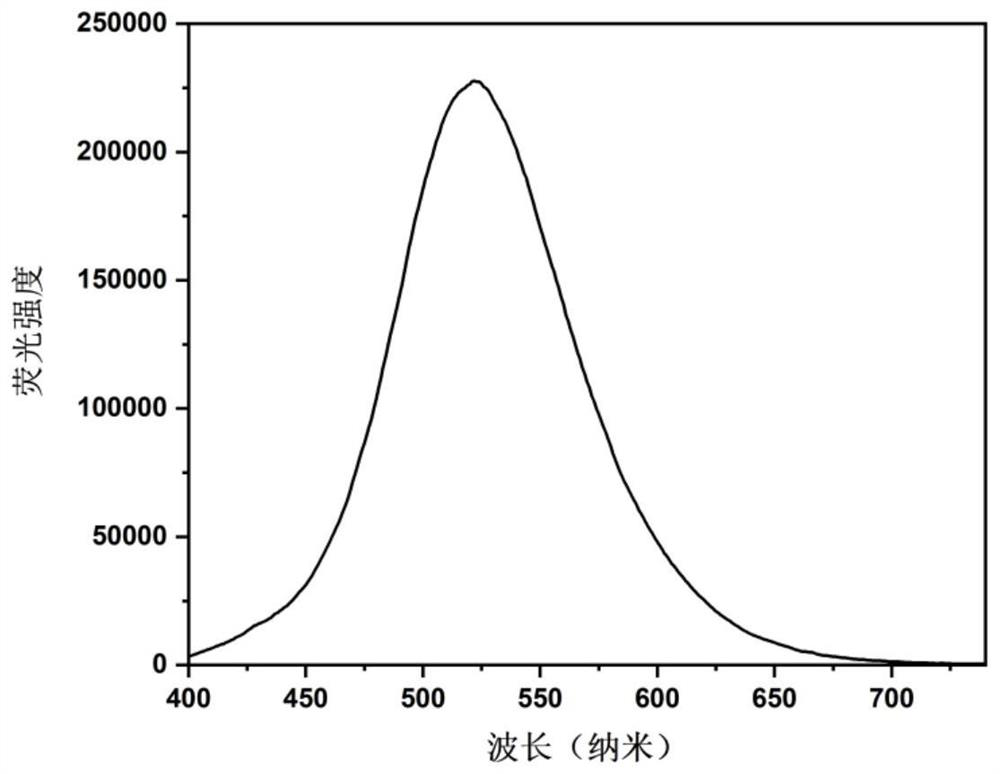
[0035] Test the fluorescence emission spectrum after the fluorescent probe prepared in Example 2 interacts with different metal ions, the specific method is as follows:
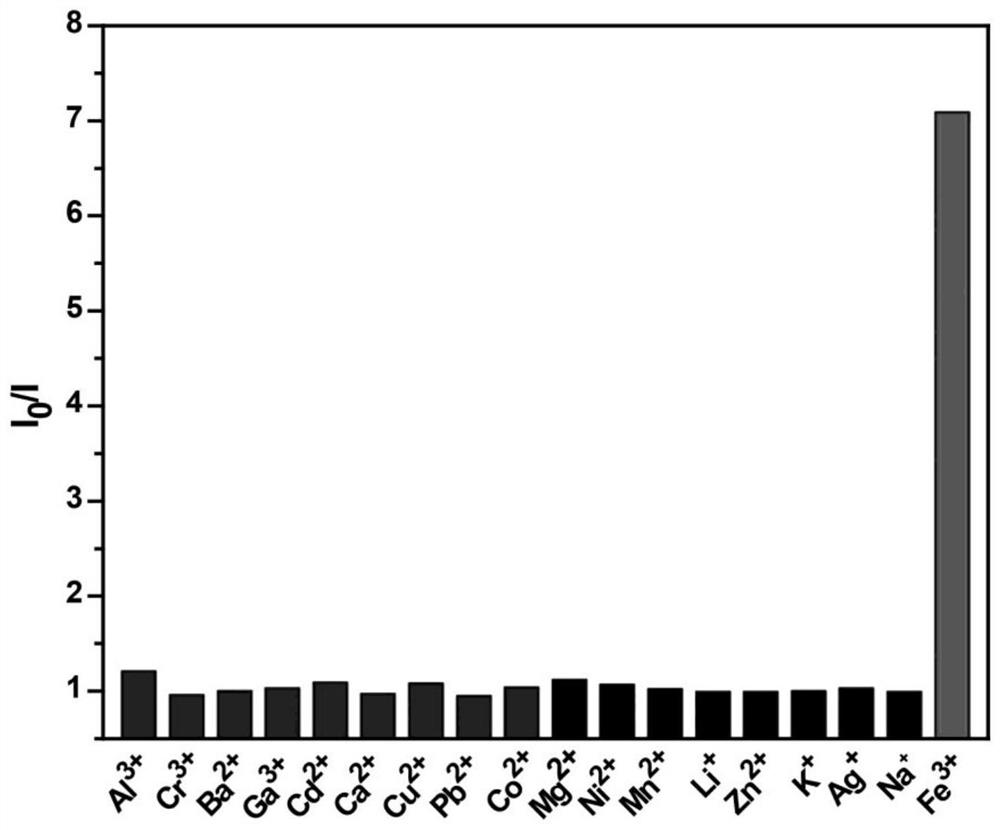
[0036] Press Fluorescent Molecular Probes D 4 H-HBC concentration is 10 -4 mol / L, the fluorescent molecular probe D 4 H-HBC was added to the ethanol solution, and then metal ions were added according to the concentration of metal ions at 5 μg / mL, and the fluorescence emission spectrum of the solution was measured, wherein the metal ions were respectively Cr 3+ , Ag + ,Co 2+ ,Cu 2+ ,K + , Ni 2+ ,Cd 2+ ,Pb 2+ , Ba 2+ ,Mg 2+ ,Al 3+ , Ca 2+ ,Zn 2+ , Na + , Li + , Ga 3+ ,Mn 2+ , these metals will not cause fluorescence quenching, when adding Fe 3+ ions, the fluorescence intensity is quenched to 1 / 7 of the original. Therefore, the fluorescent molecular probe D 4 H-HBC to Fe 3+ Have good selectivity and recognition ability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com