Medicine for inhibiting stearoyl-CoA desaturase 1
A technology of stearoyl coenzyme A and desaturase, which is applied in the field of medicine and biology to inhibit stearoyl coenzyme A desaturase 1. It can solve the problems of no parent ring structure, etc., and achieve obvious effects and obvious inhibition of enzyme activity , the effect of promoting autophagy and apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of compound
[0029] Synthesis of compound A (formula I structure):
[0030]
[0031] Add 800mg of ICT to a 250mL round bottom flask, add 150mL of acetone, 324mg of 1-bromo-3-methyl-2-butene, 300mg of anhydrous potassium carbonate, reflux at 56°C for 4 hours, cool, evaporate part of the acetone, and leave The reaction solution below was adjusted to PH to 4-5 with 1N HCl, then extracted three times with 100mL dichloromethane, and the organic layer was dried over anhydrous sodium sulfate and separated by silica gel column chromatography (petroleum ether: ethyl acetate=10:1 ) to obtain 130 mg of compound A (yield = 13.71%).
[0032] 1 H NMR (400MHz, Chloroform-d): δ12.75(s, 1H), 8.12(d, J=9.0Hz, 2H), 7.03(d, J=9.1Hz, 2H), 6.31(s, 1H), 6.12(s,1H),5.44(t,J=7.3Hz,1H),5.32(s,1H),4.58(d,J=7.3Hz,2H),3.92(s,3H),3.59(d,J =7.3Hz,2H),1.86(s,3H),1.79(s,3H),1.71(s,3H),1.65(s,3H).
[0033] Synthesis of Compound B:
[0034]
[0035] Add 800mg of...
Embodiment 2
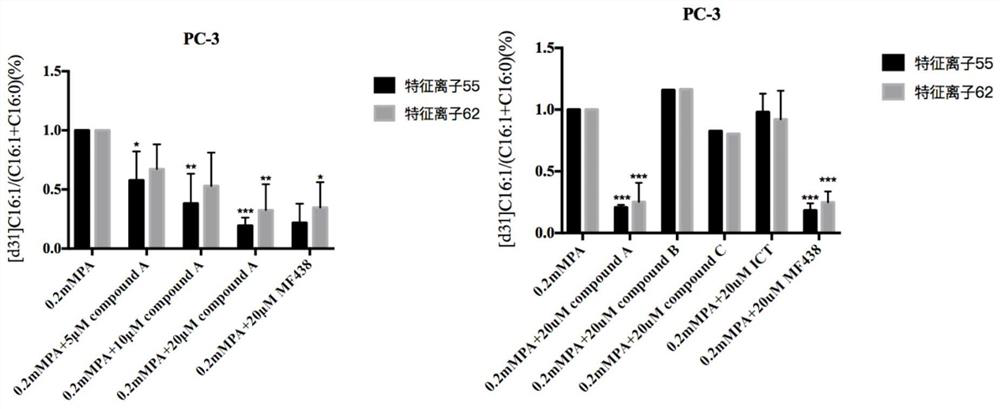
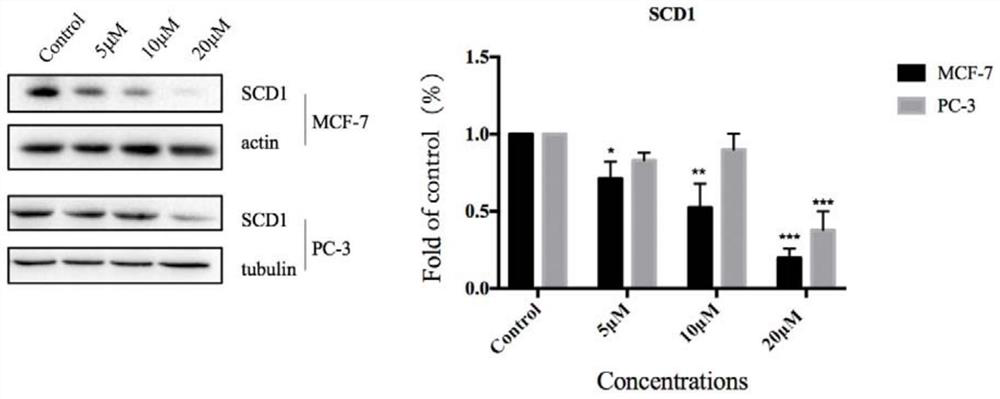
[0042] Effects of compound A, compound B, compound C, and icariin (ICT) on SCD1 enzyme activity in prostate cancer cells:
[0043] SCD1 enzyme activity detection:
[0044] (1) Collect the logarithmic phase cells, adjust the concentration of the cell suspension, and use 4x10 6 One cell / dish is appropriate.
[0045] (2) at 5% CO 2, incubate at 37°C, discard the original medium after the cells have adhered completely, and add 8 mL of 0.2 mM [d31] PA (isotope d31-labeled palmitic acid, linear molecular formula: CD 3 (CD 2 ) 14 CO 2 H) and culture medium with different concentrations of compound A, using SCD1 inhibitor MF-438 as a positive control.
[0046] (3) Discard the supernatant after incubation for 24 hours, wash twice with PBS, add 1ml CH 3 Cells were scraped off with an OH scraper and ultrasonically broken into a lipid extraction bottle.
[0047] (4) Each sample was added with 3ml CH 3 OH, 2ml CHCl 3 , 6mol / L HCl50μl, leak detection, shake for 1h. Then add 2ml C...
Embodiment 3
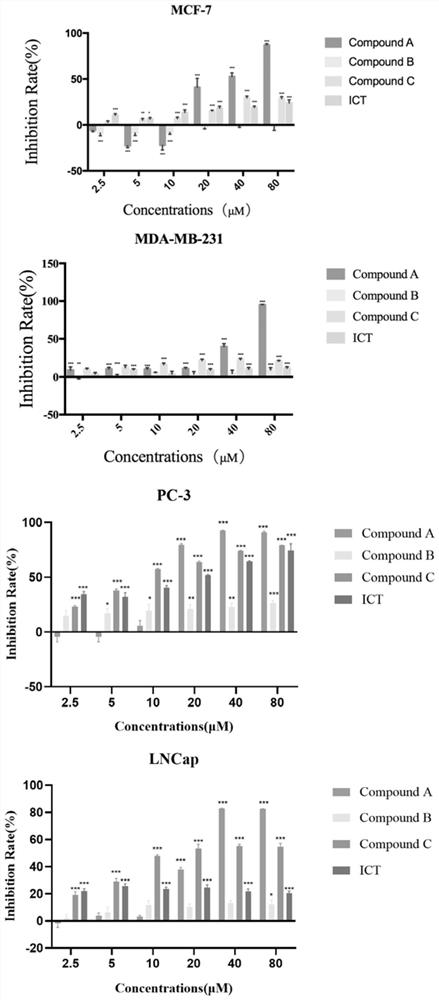
[0090] Detect the effect of compound A shown in formula I on the proliferation of tumor cells (MCF-7, MDA-MB-231, PC-3, and LNCaP):
[0091] (1) Collect the logarithmic phase cells, adjust the concentration of the cell suspension, preferably 100 μl per well of a 96-well plate, seed the plate to adjust the density of the cells to be tested to 1000-10000 per well, and fill the edge wells with sterile PBS.
[0092] (2) 5% CO 2, incubate the cells at 37°C, discard the original medium after the cells are completely attached, add 100 μl of 10% FBS medium containing 2.5, 5, 10, 20, 40, 80 μM compound A to each well, and the number of multiple wells is 4.
[0093] (3) After incubation for 24 hours, take pictures to record the state of the cells, discard the original culture medium, and add 20 μl of MTT (3-(4,5-dimethylthiazole-2)-2,5- Diphenyltetrazolium bromide, trade name: thiazolium blue).
[0094] (4) After incubation for 4 hours, discard the original culture medium, add 150 μl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com