Chiral silicon-substituted mesembrine as well as synthesis method and application thereof
A technology of escutelline and a synthesis method, which is applied in the directions of silicon organic compounds, chemical instruments and methods, drug combinations, etc., can solve the problems that a single compound cannot achieve the expected effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] A kind of synthetic method of chiral silicate mesembrine, is characterized in that, comprises the following steps:
[0127] A. 3,4-dimethoxyphenyl Grignard reagent and tetrahydrofuran solution of dichlorocyclobutane for substitution reaction, then add allylmagnesium bromide for substitution reaction to obtain compound A;
[0128] B. After dissolving S-mandelic acid and alkali and acid-binding agent triethylamine in solvent anhydrous dichloromethane, add tert-butylformyl chloride, S-mandelic acid and tert-butylformyl chloride are esterified to obtain crude product;
[0129] The crude product is condensed with propargyl alcohol under the action of the condensing agent dicyclohexylcarboimide and the catalyst 4-dimethylaminopyridine to obtain compound B;
[0130] C. Compound A and compound B carry out ring expansion reaction under the effect of catalyst divinyl rhodium dichloride dimer and ligand compound L, obtain compound C;
[0131] Wherein, the ligand compound L struct...
Embodiment 2
[0144] This embodiment provides a synthetic method of a class of chiral silamethine to further illustrate the present invention, which specifically includes the following steps:
[0145] A. Preparation of Compound A
[0146]
[0147] Add 7.2g of magnesium powder and two grains of iodine (Chengdu Jinshan Chemical Reagent Co., Ltd., 20180828) into 200mL of anhydrous tetrahydrofuran, mix well; add 3g of 3,4-dimethoxybromobenzene to the mixture, and heat to Iodine fades; then add 18.5g of 3,4-dimethoxybromobenzene, stir and react at room temperature for 30min, and obtain the newly prepared 3,4-dimethoxyphenyl Grignard reagent;
[0148] At 0°C, add the obtained 3,4-dimethoxyphenyl Grignard reagent to a tetrahydrofuran solution containing 14.1 g of dichlorocyclobutylsilyl, react at room temperature for 24 h, and then add 120 mL of Allylmagnesium bromide was reacted at room temperature for 24h; then, it was quenched with 100mL of saturated ammonium chloride solution, diluted with...
Embodiment 3
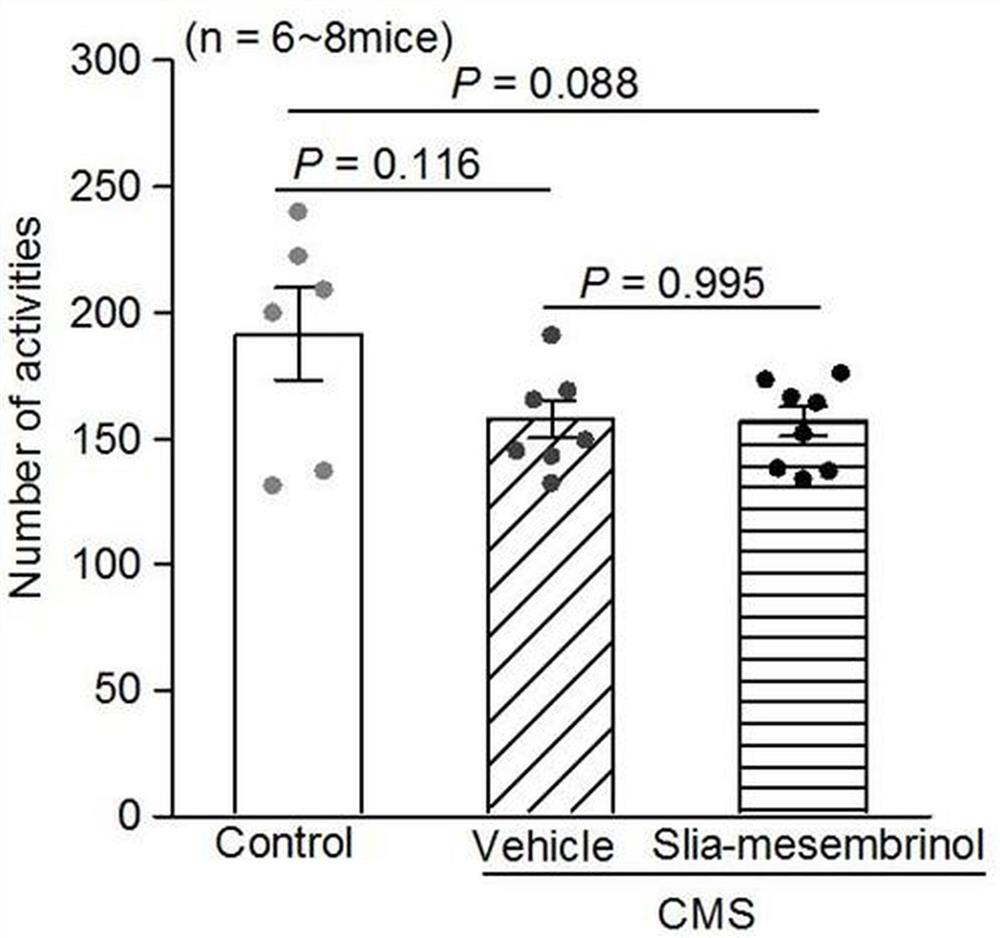
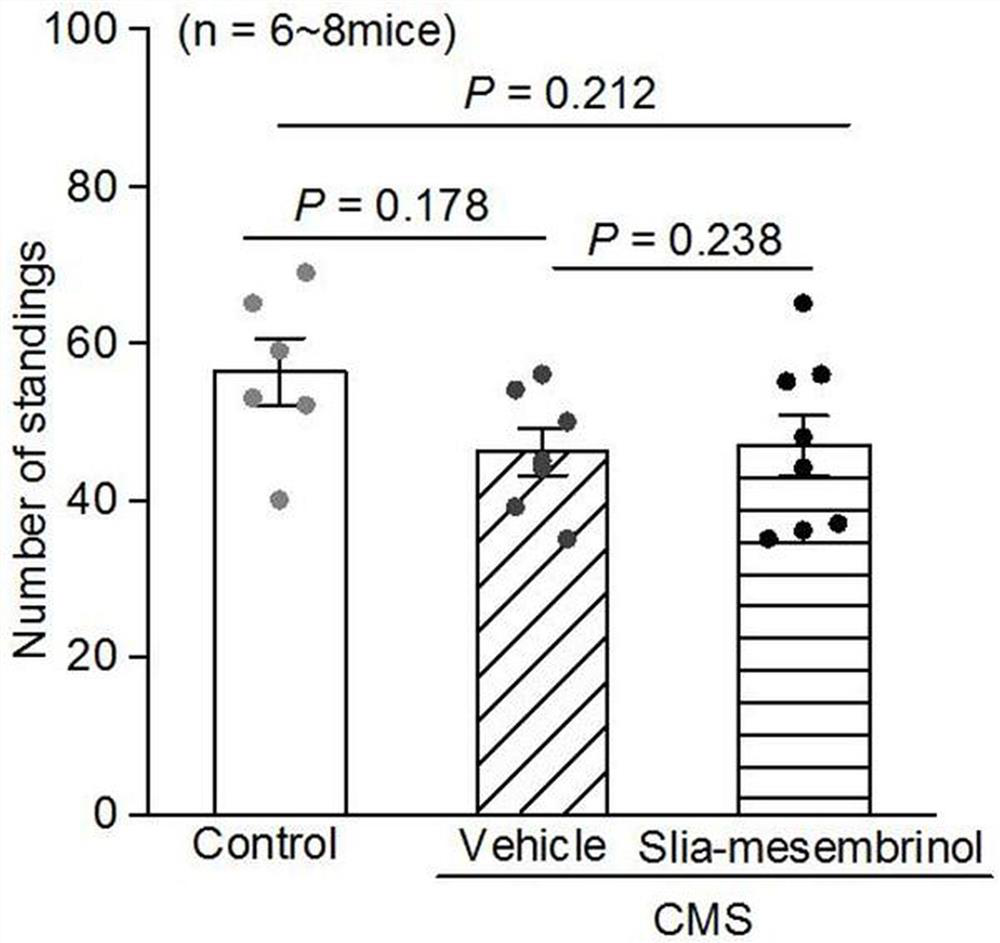
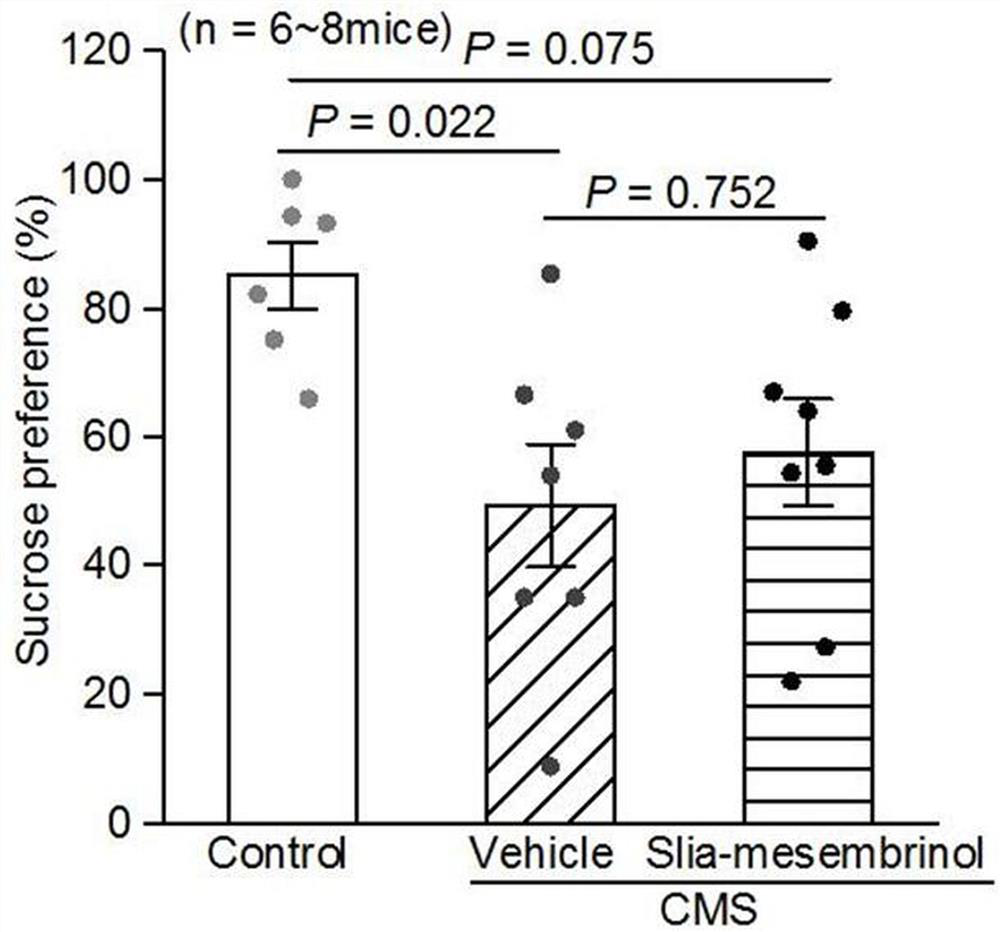
[0191] Based on Example 2, this example studies the activity of the obtained chiral silicate mesembrine I-a to further illustrate the present invention.
[0192] 1. Animals
[0193] 8-week-old C57BL / 6 male mice (purchased from Chengdu Dashuo Experimental Animal Co., Ltd.) were bred. During the feeding, fluorescent lamps were used to maintain light for 12 hours a day, and the room temperature was kept at 22°C; the mice were given sufficient feed and drinking water (all experimental animals All are implemented in accordance with the provisions on the protection of experimental animals);
[0194] 2. Drugs
[0195] It is prepared from silicene compound Ⅰ-a and sulfobeta cyclodextrin aqueous solution (the mass fraction of sulfobeta cyclodextrin is 20%);
[0196] 3. Establishment of chronic mild stress model in mice
[0197] 8-week-old C57BL / 6 male mice were subjected to continuous chronic mild stress for 4 weeks, and 3 different stresses were given every day, and the stresses we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com