Thermostable epidemic encephalitis B vaccine soluble microneedle delivery system
A delivery system and thermally stable technology, which can be applied in the direction of microorganisms, drug delivery, and resistance to vector-borne diseases. It can solve the problems of small drug loading, harmful residues, and difficulty in controlling the dosage of coated microneedles, and achieve vaccine stability. Excellent performance, good thermal stability, and good mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 JE vaccine soluble microneedle preparation
[0023] The JE vaccine soluble microneedle to be prepared in the present invention includes a needle body and a backing, and the needle body is composed of the JE vaccine, a vaccine stabilizer and a suitable matrix material.
[0024] The preparation method of Japanese encephalitis vaccine soluble microneedle of the present invention is as follows:
[0025] The Japanese encephalitis vaccine, the vaccine stabilizer and the matrix material are mixed according to the prescription ratio, and deionized water is added to fully dissolve it to form a uniform needle body liquid. Take an appropriate amount of needle body liquid and pour it into the polydimethylsiloxane female mold, so that the needle body liquid is completely immersed in the hole of the female mold, and then put it into a high-speed centrifuge, set the speed at 4000r / min, and centrifuge for 5 minutes to make the microneedle solution fully Enter the female m...
Embodiment 2
[0026] Example 2 Relevant tests of JE vaccine soluble microneedles
[0027] 1. Characterization method of JE vaccine stability
[0028] The prepared microneedles were dissolved in 10 mL of deionized water, and the vaccine antigen content in the solution was detected by ELISA (enzyme-linked immunosorbent assay) method, which was recorded as the initial 0-month vaccine antigen content (vaccine stability was regarded as 100%) In addition, the various microneedles prepared in the same batch as above were stored in an environment with a temperature of 37°C for 1 month, and then the same ELISA method was used to detect the percentage of the antigen content of each microneedle vaccine relative to the initial time, and recorded is the percentage of vaccine antigen content.
[0029] When the Japanese encephalitis vaccine is stored in an environment with a temperature of 37°C for 1 month, the percentage of vaccine antigen content is still higher than 70%, which is considered to meet th...
Embodiment 3
[0036] The type of vaccine stabilizer of embodiment 3 is investigated on the influencing factors of vaccine stability
[0037] Set the mass ratio of microneedle matrix material and JE vaccine to a certain value, only change the type of vaccine stabilizer, and explore the influence of different vaccine stabilizers on the protective effect of JE vaccine in soluble microneedles.
[0038] The type of vaccine stabilizer is selected as one or more combinations of human albumin, polyvinylpyrrolidone (PVP), arginine, trehalose, and dextran, corresponding to experimental groups 2-11.
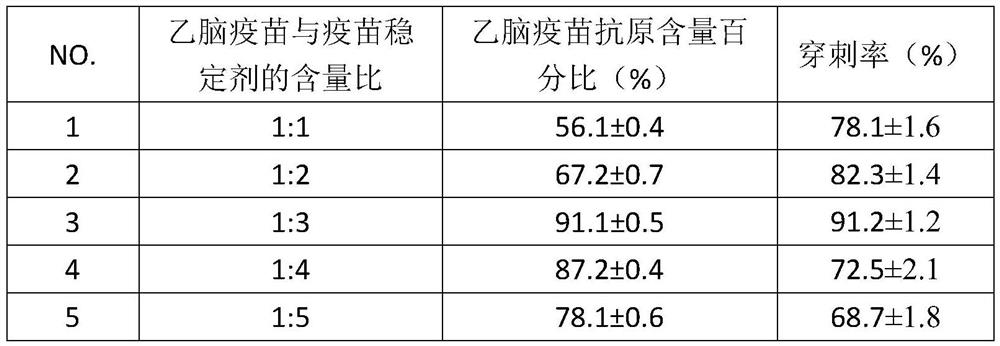
[0039] In this example, the preparation process of JE vaccine soluble microneedles is the same as Example 1. The test method for the stability of JE vaccine is shown in Example 2, and the test results are shown in Table 1.
[0040] Table 1. The impact of the type of vaccine stabilizer on vaccine stability (mean%±SD%, n=6)
[0041] No. vaccine stabilizer Antigen content percentage of JE vac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com