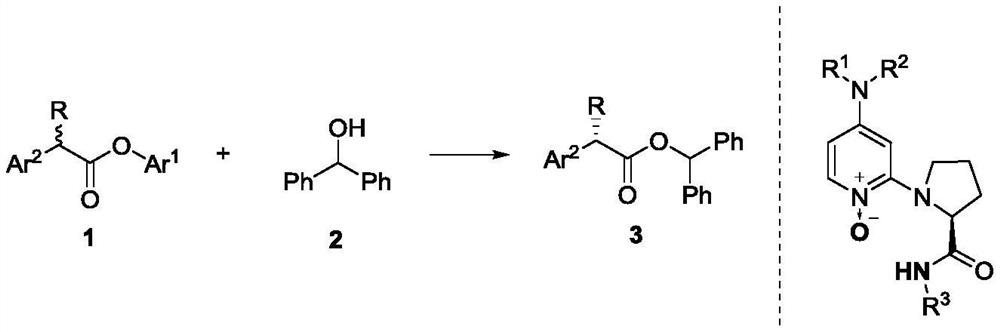
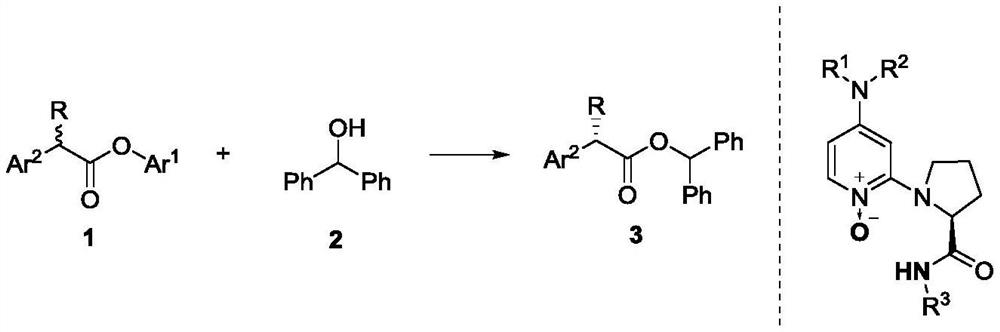
Method for dynamic kinetic resolution of alpha-aryl-alpha-alkyl carboxylic ester and application
A dynamic kinetics, technology of alkyl carboxylate, applied in the direction of organic chemical method, carboxylate/lactone preparation, reaction preparation of ester group and hydroxyl group, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024]
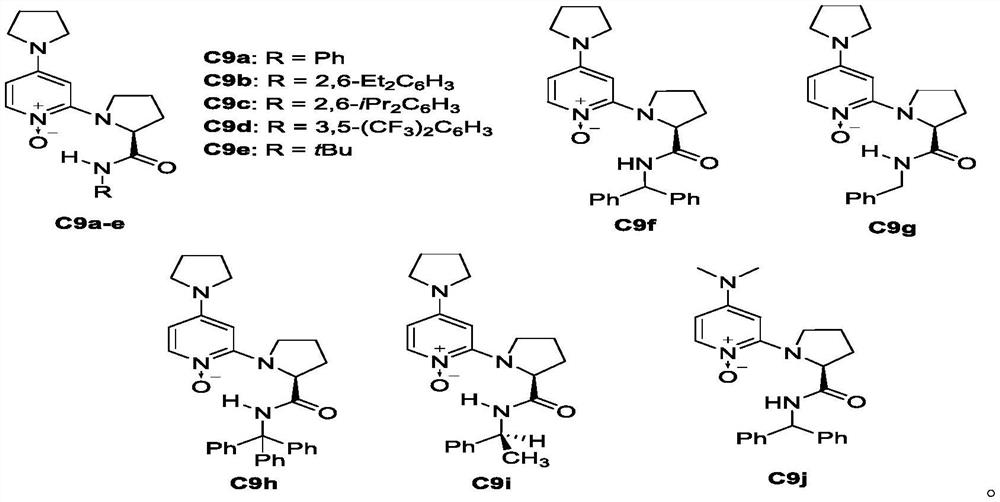
[0025] [a] Unless otherwise stated, the reaction conditions are as follows: ester (0.05mmol), 2a (18.4mg, 0.1mmol), catalyst C9f (2.2mg, 0.005mmol, 10mol%), iPr 2 EtN (17.4 μL, 0.1 mmol) was placed in DCM (0.5 mL) for 72 hours. [b] NMR yield. [c] Determined by chiral HPLC analysis. N.R = no response.
[0026] Taking pentafluorophenol ester 1b and benzhydryl alcohol 2a as raw materials to generate 3a as an example, the reaction conditions were optimized, and the reaction equation was as follows:
[0027]
[0028] The specific reaction results are shown in the following table:
[0029]
[0030] [a] Reaction conditions: 1 (0.05mmol), 2a (0.1mmol), catalyst (10mol%) and base (2equiv) react in solvent (0.5mL) for 72h. [b] NMR yield. [c] Determined by chiral HPLC analysis. [d] PhCF 3 / DCM(1 / 1,v / v).[e]Et 3 N(5equiv), PhCF 3 / DCM(1 / 1,v / v).[f]1b(0.2mmol),2a(0.4mmol),C9f(10mol%),andEt 3 N(5equiv)in PhCF 3 / DCM (0.5mL, 1 / 1, v / v) for 72h. [g] C9f (5...
Embodiment 2
[0034] In a dry 5mL reaction tube, add chiral catalyst C9f (8.8mg, 0.02mmol, 10mol%), benzhydryl alcohol 2a (73.6mg, 0.4mmol) and pentafluorophenol ester 1b-l (0.2mmol) respectively, and then Trifluorotoluene / dichloromethane (1 / 1, v / v, 0.5 mL) was added, and finally triethylamine (152 μL, 1 mmol) was added, and the reaction solution was stirred at 0° C. for 72 hours. After the reaction was completed, the product was obtained after column chromatography.
[0035] The specific results are as follows:
[0036]
[0037]
[0038] Representative NMR characterization data are as follows:
[0039] (S)-Benzhydryl-2-(o-tolyl) propanoate (3c)
[0040]
[0041] Colorless oil, 55.5mg, 84% yield, 95% ee; R f=0.38(Pet / EtOAc, 10 / 1, v / v).HPLCCHIRALCEL ID, n-hexane / 2-propanol=90 / 10, flow rate=0.6mL / min, λ=256nm, retention time: 9.060min(minor ),9.478min(major).[α] D 21 =+33.4 (c=2.0, CHCl 3 ); 1 HNMR (400MHz, CDCl 3 )δ7.32–7.22(m,5H),7.21–7.09(m,7H),7.06–7.00(m,2H),6.83(s,1H),...
Embodiment 3
[0061]
[0062] In a dry 5mL reaction tube, add chiral catalyst C9f (8.8mg, 0.02mmol, 10mol%), benzhydryl alcohol 2a (73.6mg, 0.4mmol) and pentafluorophenol ester 1m (74.4mg, 0.2mmol), Then trifluorotoluene / dichloromethane (1 / 1, v / v, 0.5 mL) was added, and finally triethylamine (152 μL, 1 mmol) was added, and the reaction solution was stirred at 0° C. for 72 hours. Column chromatography separated to obtain 66.2mg of colorless oil 3m, yield 89%, 90% ee. HPLC CHIRALCEL IA, n-hexane / 2-propanol=99 / 1, flow rate=0.5mL / min, λ=256nm, retention time: 27.917min(major), 33.453min(minor)[α] D 21 =+20.5 (c=1.40, CHCl 3 ). 1 H NMR (400MHz, CDCl 3 )δ7.32-7.21(m,5H),7.21-7.14(m,5H),7.11-7.03(m,4H),6.81(s,1H),3.80(q,J=7.2Hz,1H),2.46 (d,J=7.2Hz,2H),1.89-1.82(m,1H),1.51(d,J=7.2Hz,3H),0.91(d,J=7.2Hz,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com