A kind of preparation method of phosphorus and selenium co-doped niobium disulfide nanomaterials
A niobium disulfide, nanomaterial technology, applied in chemical instruments and methods, nanotechnology, niobium compounds, etc., to achieve the effect of continuous doping process, huge application value, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of phosphorus and selenium co-doped niobium disulfide nanoflowers
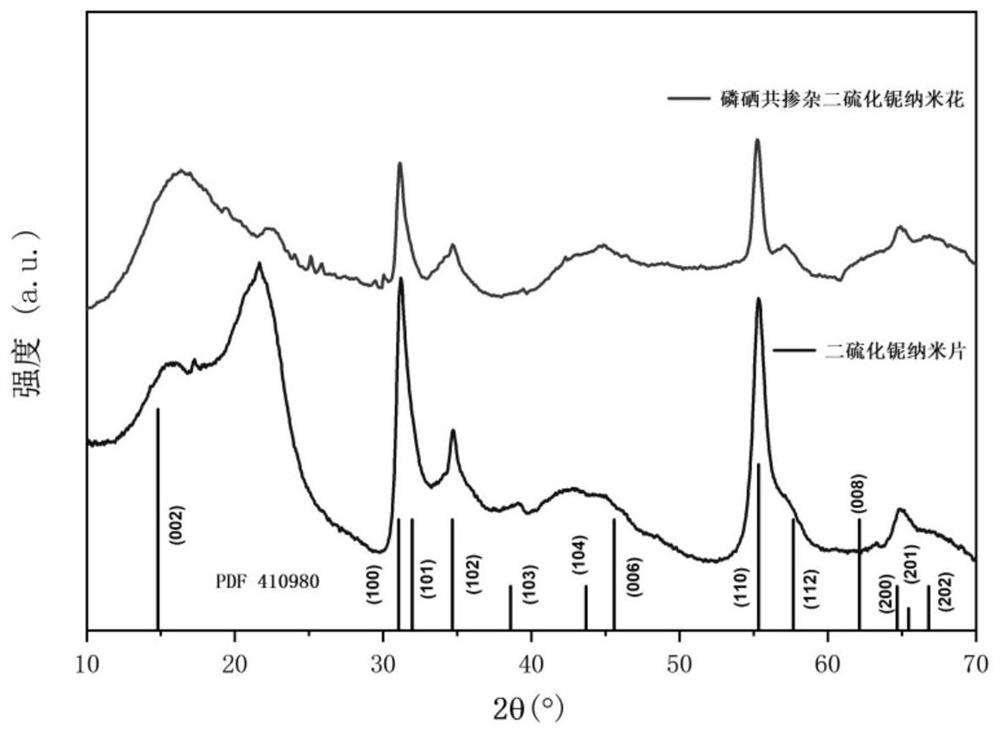
[0036] 4mL of oleylamine was put into a three-necked flask for magnetic stirring, heated to 130°C under the protection of nitrogen, kept for 20 minutes, then naturally cooled, 1.6mmol of niobium pentachloride and degassed oleylamine were placed in the three-necked flask. Mixed in medium and magnetically stirred, heated to 300 °C under the protection of nitrogen, injected with 8 mmol CS 2 The sulfur source was kept at this temperature for 2 hours, and then naturally lowered to 200 °C. The TOP-Se (1mmol-0.3mmol) solution prepared in advance was injected, and the temperature of the system was rapidly increased to 320 °C. After 1 hour, the reaction ended. , the solution was naturally cooled to room temperature, washed with n-hexane and methanol, and then freeze-dried. like figure 1 As shown, the X-ray diffraction pattern (XRD) of the phosphorus-selenium co-doped niobium disulfide nan...
Embodiment 2 2
[0037] Example 2 Preparation of niobium disulfide nanosheets
[0038] 4 mL of oleylamine and 1.6 mmol of niobium pentachloride were placed in a three-necked flask for magnetic stirring. 2 The sulfur source was kept at this temperature for 3 hours, then the temperature was naturally cooled, the solution was naturally cooled to room temperature, the samples were washed with n-hexane and methanol, and then freeze-dried. Figures 6 to 7 Scanning electron micrographs with a scale bar of 3 μm and 2 μm, respectively, are distinct from the nanoflowers and are uniformly distributed. Figures 8 to 9 Transmission electron micrographs with scale bars of 500 nm and 200 nm, respectively. It is clearly seen that NbS 2 Nanosheets and NbS 2 Nanoflowers have a smaller diameter, around 200 nm.
[0039] Surface scanning was carried out on the phosphorus-selenium co-doped niobium disulfide nanoflowers and niobium disulfide nanosheets prepared in Example 1 and Example 2, through Figures 10 to...
Embodiment 3
[0040] Example 3 Electrocatalytic hydrogen evolution performance test
[0041] The electrochemical catalytic properties of phosphorus and selenium co-doped niobium disulfide nanoflowers and niobium disulfide nanosheets were measured using an electrochemical workstation. like Figure 20As shown, for the co-doped niobium disulfide nanoflowers with phosphorus and selenium, the niobium disulfide nanosheets in the acidic solution of 0.5MH 2 SO 4 Linear sweep voltammetry and Tafel slope curves in . It can be seen that after the control of the morphology and the influence of phosphorus and selenium co-doping, at 10 mA cm -2 The overpotential increases from -0.534 V for niobium disulfide nanosheets to -0.368 V for phosphorus-selenium co-doped niobium disulfide nanoflowers at a current density of The current density of nanoflowers can reach 130 mA cm -2 , while at the same overpotential, the current density of the niobium disulfide nanosheets is only 13.8 mAcm -2 . like Figure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com