GLP-1R/GIPR dual-target agonist fusion protein as well as preparation method and application thereof
A GLP-1R, fusion protein technology, applied in the field of biopharmaceuticals, can solve the problems such as no reports related to dual-target agonist fusion proteins, avoid downstream denaturation and renaturation processing, high hypoglycemic activity, hypoglycemic long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Design of GLP-1R / GIPR dual-target agonist long-acting fusion protein
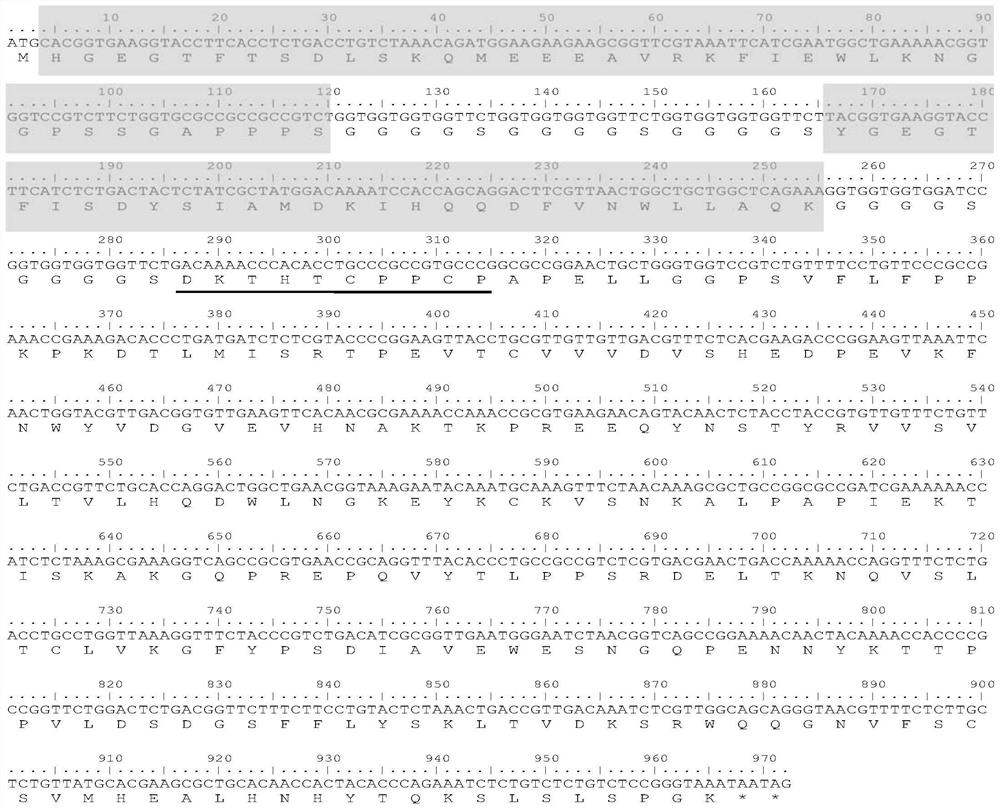
[0040] GLP-1R / GIPR dual-target agonist long-acting fusion protein structure such as figure 1 As shown, from the N-terminal to the C-terminal, it consists of the following polypeptide domains in series: highly active exenatide mutant (EX-L21K), Gly-rich linker peptide (GGGGS) 3, human glucose-dependent insulinotropic polypeptide (GIP) mutant (GIP-A2G), Gly-rich connecting peptide (GGGGS) 3, optimized mutant human IgG1 hinge region DKTHTCPPCP (underlined), human IgG1 constant region CH2-CH3.
[0041] Among them, the highly active exenatide mutant EX-L21K was obtained by mutating the 21st position of wild-type exenatide from Leu to Lys.
[0042] The GIP mutant GIP-A2G was obtained by mutating the second Ala of natural GIP to Gly.
[0043] The hinge region of optimized mutated human IgG1: has the amino acid sequence shown in -DKTHTCPPCP- (SEQ ID NO: 8).
[0044] Human IgG1 constant region CH...
Embodiment 2
[0046] Example 2 Cloning and expression of GLP-1R / GIPR dual-target agonist long-acting fusion protein
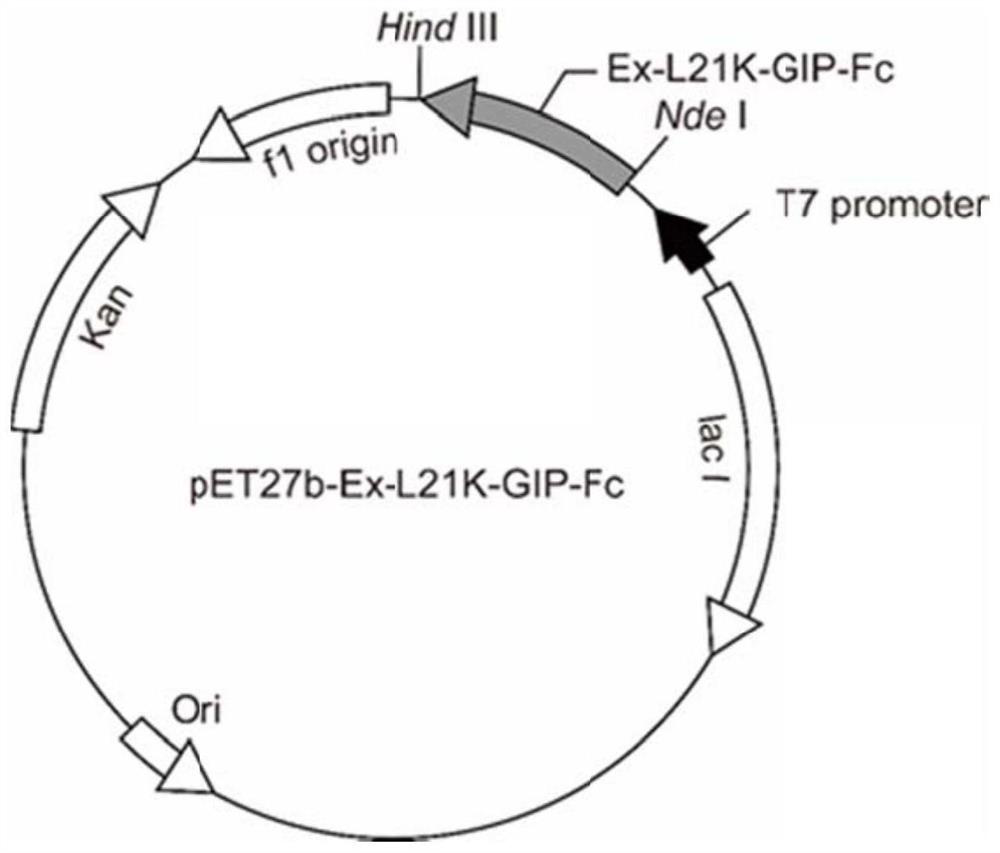
[0047] (1) Construction of recombinant plasmid pET27b-IgG1Fc
[0048] Using the plasmid containing the human IgG1Fc coding gene (SEQ ID NO: 16) synthesized in the laboratory as a template, design the following forward and reverse primers (the underlines indicate the restriction sites of BamH I and HindIII respectively):
[0049] BamHI-IgG1Fc-F: AATT GGATCC GGTGGTGGTGGTTCTGACAAAACCCACACCTGC (SEQ ID NO: 17);
[0050] HindIII-IgG1Fc-R: GGCCGC AAGCTT CTATTATTTACCCGGA (SEQ ID NO: 18).
[0051] The above primers were synthesized by Nanjing GenScript Biotechnology Company.
[0052] The IgG1Fc gene fragment was amplified by PCR technology, digested with BamH I and Hind III, and inserted into the corresponding restriction site of the expression vector pET27b to obtain the recombinant plasmid pET27b-IgG1Fc containing the gene encoding human IgG1Fc.
[0053] (2) Construction of ...
Embodiment 3
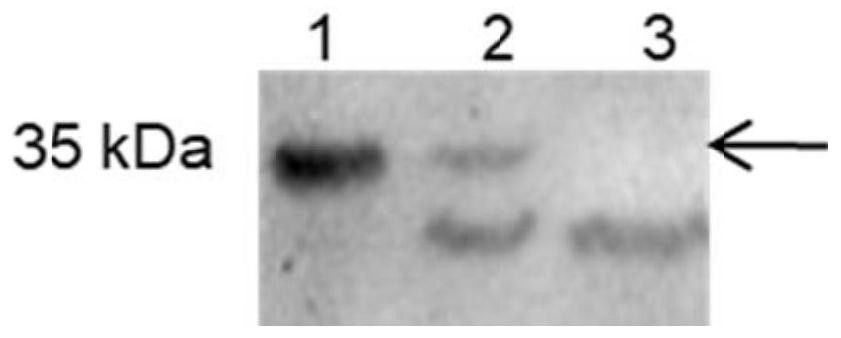
[0058] Embodiment 3 fusion protein EX-L21K-GIP-Fc engineering bacteria fermentation and separation and purification
[0059] (1) Preparation of seed solution
[0060] Glycerol tubes were used to pick up the bacterial liquid, and two three-section line cultures were carried out on the LBK culture plate. Pick a single colony from the LBK plate, insert it into 30mL LBK liquid medium, and cultivate it at 37°C and 220rpm for about 12h. This is the primary seed solution. Transfer the primary seed solution to two 500mL Erlenmeyer flasks with 100mL LBK liquid medium at 37°C with an inoculum size of 2% (v / v), and cultivate it at 220rpm for 12h. This is the secondary seed solution for high-density fermentation ,
[0061] (2) Fed-batch fermentation
[0062] The secondary seed solution was inserted with 4% (v / v) inoculum amount containing 4.5L fermentation medium (yeast powder 2.4% (w / v), tryptone 1.2% (w / v), glycerol 0.4% ( v / v), 17mM KH2PO4, 72mM K2HPO4 3H 2 O, defoamer 0.1% (v / v),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com