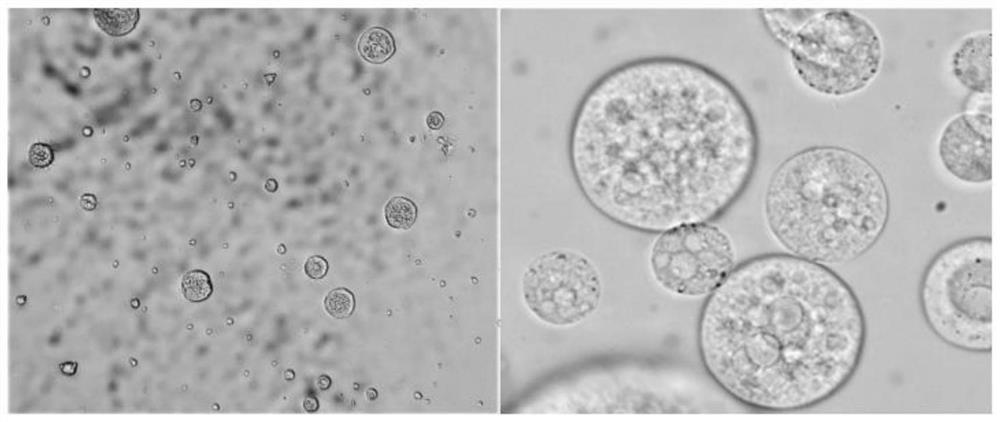
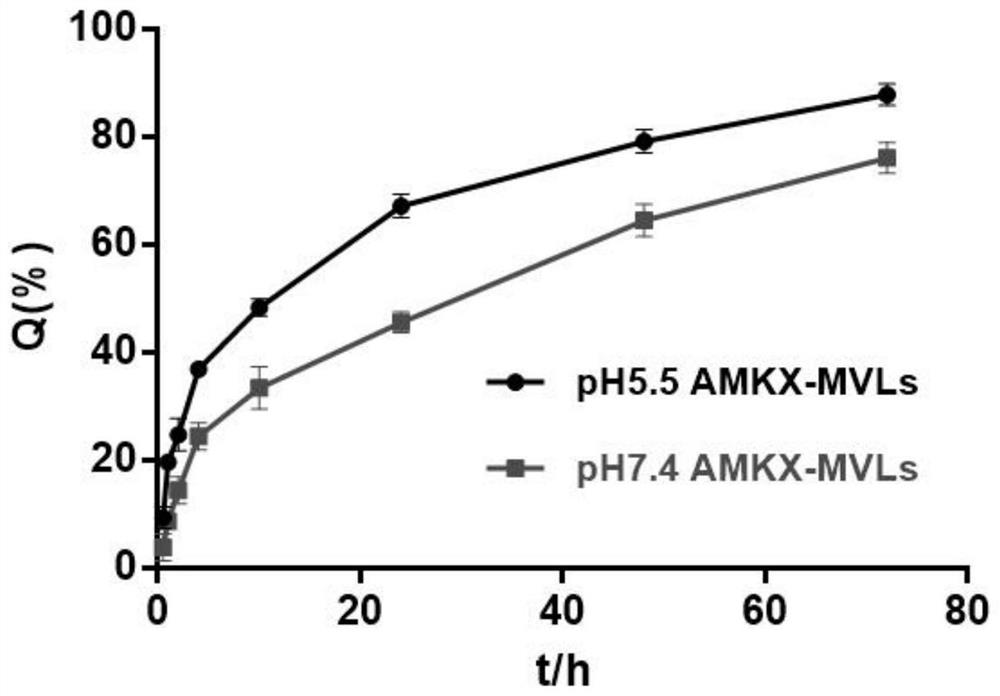
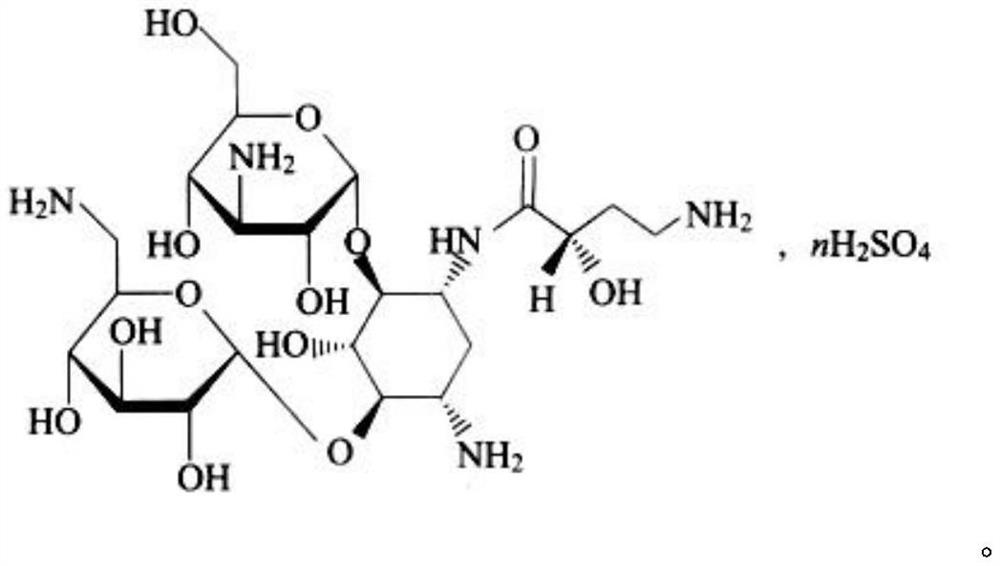
Amikacin sulfate multivesicular liposome for local injection and preparation method of amikacin sulfate multivesicular liposome
A technology of amikacin sulfate and multivesicular liposome, which is applied in the field of amikacin sulfate multivesicular liposome for local injection and its preparation, can solve the problem of difficult to kill living bacteria, poor antibiotic treatment effect, and chronic infection Recurrent infection and other problems, to achieve the effect of improving bioavailability, good antibacterial treatment effect, and reducing the toxicity of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Amikacin sulfate multivesicular liposomes, including the raw material formula in the following table 1: in combination with literature information and drug dosage, the fixed main drug concentration is 50mg / mL, and the SPC:CH:TO:ODA quality in the lipid material The ratio is 4:3:2:1, and the changes in the appearance, particle size and encapsulation efficiency of this product are investigated when the drug-to-lipid ratio is 3:1, 2:1, and 1:1.
[0046] Table 1
[0047]
[0048]
[0049] The preparation process is as follows:
[0050] Each group of samples was dissolved in 3ml chloroform of SPC, CH, TO, ODA respectively, as the oil phase for later use; Amikacin sulfate was dissolved in 3ml 7% sucrose solution, as the inner water phase for later use; 625mg glucose Dissolve in 12.5ml of 0.5% PVA (polyvinyl alcohol) solution, and use it as the outer water phase for later use; disperse the inner water phase into the oil phase, stir at 10000r / min for 5min, and then ultra...
Embodiment 2
[0053] Combining with literature reports and pre-experimental results, this example elaborates in detail stearylamine as a membrane stabilizer, whether it is added to the formula and the effect of its dosage on the appearance, particle size and encapsulation efficiency of this product.
[0054] Amikacin sulfate multivesicular liposomes, including the raw material formula of following table 2:
[0055] Table 2
[0056]
[0057] The preparation process is as follows:
[0058] For each group of samples, dissolve SPC, CH, TO, and ODA in 3ml chloroform respectively, and use it as an oil phase; dissolve amikacin sulfate in 3ml 7% sucrose solution, and use it as an internal water phase; Glucose was dissolved in 20ml of 0.5% PVA solution and used as the external water phase for later use; the internal water phase was dispersed into the oil phase, stirred at 10,000r / min for 5min, and ultrasonically disrupted for 4min in an ice bath (power 300W) to form a stable W / O-type colostrum...
Embodiment 3
[0061] Amikacin sulfate multivesicular liposomes, including the raw material formula of following table 3:
[0062] table 3
[0063]
[0064] The preparation process is as follows:
[0065] Two groups of samples were respectively dissolved in 3ml of chloroform-ether mixture (v / v, 1:1) in the SPC, CH, TO, ODA of formula quantity, as the oil phase standby; Amikacin sulfate was dissolved in 3ml of 7% The sucrose solution is used as the inner water phase for later use; 1 g of glucose is dissolved in 20 ml of 0.5% PVA solution, and it is used as the outer water phase for later use. For sample 8, disperse the inner water phase into the oil phase, stir at 10000r / min for 9min, draw colostrum into 12.5ml of outer water phase at a certain speed with a syringe, vortex mix for 10s to form a double emulsion, and transfer the double emulsion to an open glass Place the bottle in a water bath at 37±0.2°C, stir at a certain speed and blow with nitrogen for 10-15 minutes, remove the organi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com