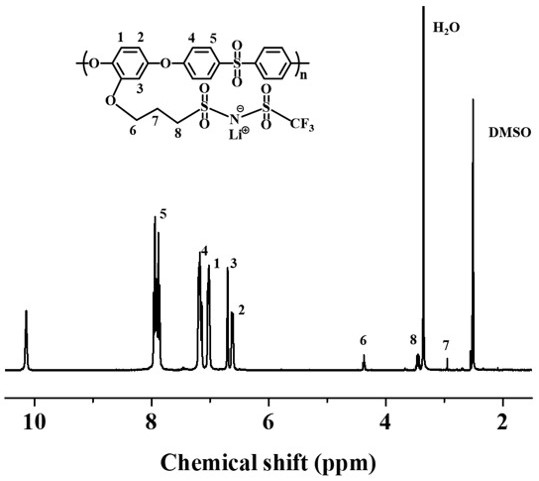
Polyethersulfone single-ion polymer and single-ion gel polymer electrolyte
A gel polymer and ionic polymer technology, applied in the field of single-ion gel polymer electrolyte, can solve problems affecting battery stability, affecting battery performance, accelerating lithium dendrite growth, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
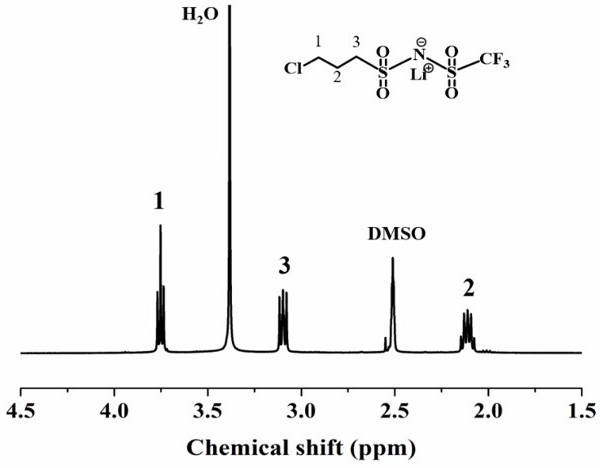
[0053] Example 1 Preparation of lithium 3-chloropropanesulfonyl (trifluoromethanesulfonyl)imide (LiCPSI)
[0054] Under a nitrogen atmosphere, add 20 mmol (2.9818 g) of trifluoromethanesulfonamide and 40 mmol (0.9572 g) of LiOH into 20 ml of anhydrous acetonitrile, and add 20 mmol (3.541 g) of ) of 3-chloropropanesulfonyl chloride. Subsequently, the reaction was carried out at room temperature for 24 h, acetonitrile was removed by rotary evaporation, and the obtained solid lithium 3-chloropropanesulfonyl(trifluoromethanesulfonyl)imide (LiCPSI) was recrystallized in dichloromethane, and 7.4125 g of white solid was obtained by filtration.
Embodiment 2
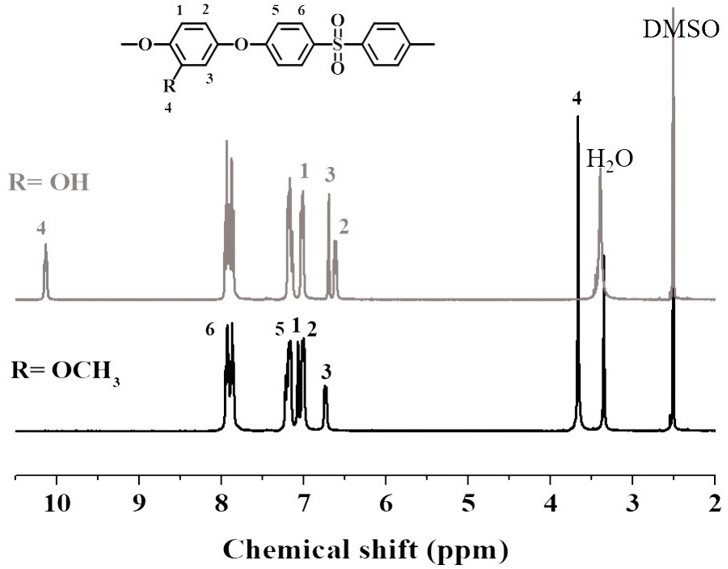
[0055] Example 2 Containing methoxy polyethersulfone (PES-OCH 3 ) preparation
[0056] In a three-necked flask equipped with a mechanical stirrer, a nitrogen inlet tube, a thermometer, and a water separator, add 5.6056 g of 2-methoxyhydroquinone (MHQ) and 10.17 g of 4,4'-difluorodiphenyl sulfone in sequence (FPS), 6.624 g of anhydrous potassium carbonate, 50 ml of sulfolane (TMS), and 18 mL of toluene were heated to 140-170 °C for 12 h under stirring with nitrogen gas to obtain a viscous solution, and then the viscous solution was added to distilled water. After cooling, the product is pulverized, washed with deionized water and ethanol, and dried to obtain methoxy-containing polyethersulfone (PES-OCH 3 ).
Embodiment 3
[0057] Example 3 Preparation of hydroxyl-containing polyethersulfone (PES-OH)
[0058] In a three-necked flask equipped with a mechanical stirrer, a nitrogen inlet tube, a thermometer, and a water separator, the PES-OCH obtained in 6 g of Example 2 was 3 Dissolve in anhydrous chloroform, add 60 ml of chloroform solution containing 10% boron tribromide dropwise at -40 °C, react at room temperature for 12 h, sink into ethanol, wash with deionized water and ethanol, and dry to obtain Hydroxypolyethersulfone (PES-OH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com