Novel coronavirus nucleic acid PCR-colloidal gold immunochromatography detection kit
A technology of immunochromatography and detection kit, which is applied in the field of novel coronavirus nucleic acid colloidal gold immunochromatography detection kit, can solve problems such as inability to effectively monitor the nucleic acid detection process, inability to set internal reference gene detection, and contamination. Achieve the effects of saving detection time, high clinical applicability, and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
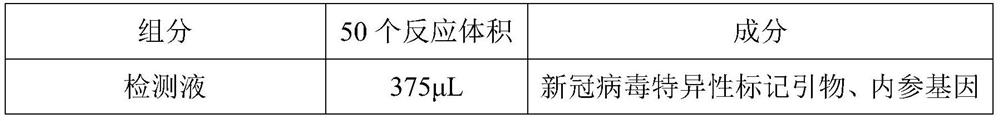
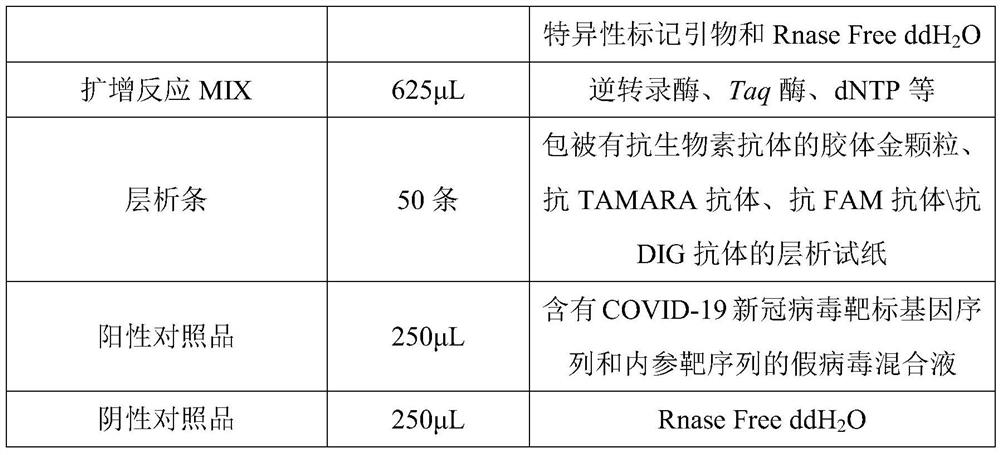
[0036] A novel coronavirus nucleic acid PCR-colloidal gold immunochromatography detection kit, comprising: amplification reaction solution (i.e. amplification reaction MIX, purchased from Nanjing Nuoweizan Biotechnology Co., Ltd. (article number: Q222-CN), the The reaction system includes reverse transcriptase, Taq DNA polymerase, 4 kinds of dNTP and various ions required for PCR amplification reaction solution), CoV detection solution, positive control (mixture of virus target gene plasmid and internal reference plasmid), negative control product (RNase Free ddH2O) and chromatographic strip (coated with colloidal gold particles and antibodies); the CoV detection solution includes specific marker primers for the new coronavirus open reading frame 1ab and the gene encoding the nucleocapsid protein N, internal reference genes RNase P-specific labeled primers and RNase Free ddH 2 O.
[0037] The primer sequences in this example are as follows:
[0038] New coronavirus amplifica...
Embodiment 2
[0073] Performance verification of the kit described in embodiment 1
[0074] (1) Sample processing
[0075] Select the national reference product of the new coronavirus nucleic acid detection reagent purchased to verify the performance of the kit, take the sample to be tested into a 1.5mL centrifuge tube, use the nucleic acid extraction or purification reagent produced by Tiangen Biochemical Technology (Beijing) Co., Ltd., and strictly follow the instructions. RNA extraction operation. Extracted RNA samples should be tested immediately or stored below -70°C and avoid repeated freezing and thawing.
[0076] (2) PCR amplification
[0077] Configure the PCR amplification reaction solution (20 μL for each reaction) according to Table 3.
[0078] Table 3 PCR amplification reaction solution
[0079] components 1 reaction volume CoV detection solution 7.5μL Amplification reaction solution 12.5μL total capacity 20 μL
[0080] Aliquot the prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com