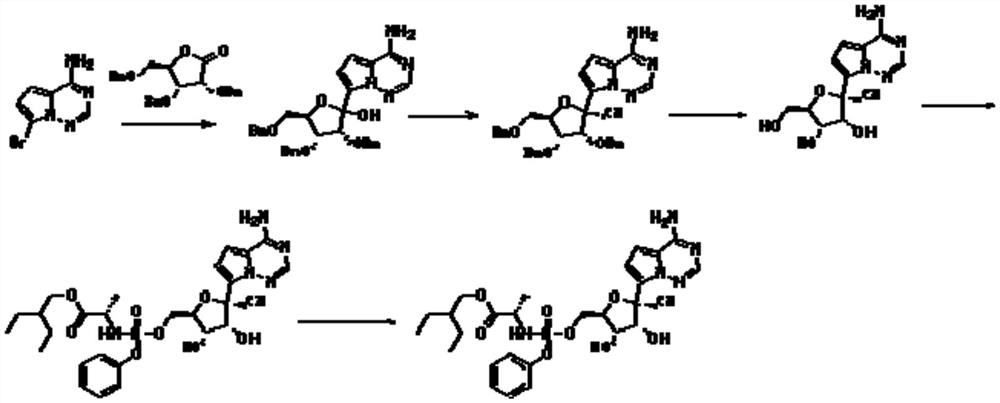
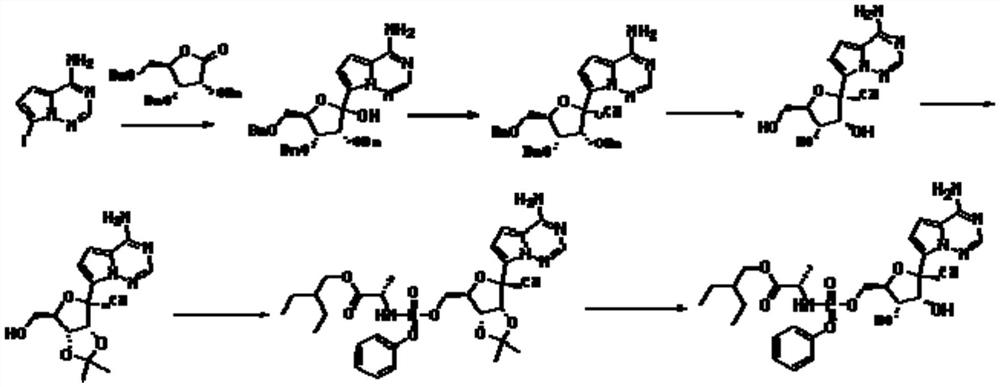
Preparation method of ridecevir compound
A technology of remdesivir and compounds, which is applied in the field of new synthesis and preparation of remdesivir compounds, can solve problems such as unsuitable for industrial scale-up, high cost, and low yield, and achieve high cost, low environmental pollution, and low yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
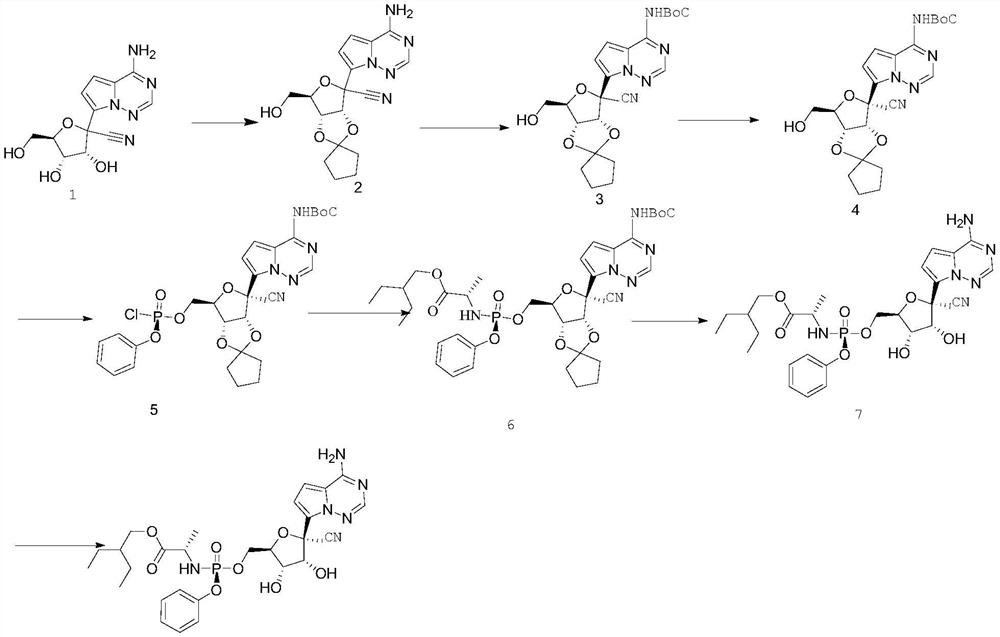
[0025] A preparation method of remdesivir compound, the method uses compound 1 as starting material,
[0026]
[0027] Include the following steps:
[0028] a) compound 1 generates compound 2 through cyclization reaction,
[0029]
[0030] The reaction is carried out in the solvent cyclopentanone. Compound 1 is mixed with p-toluenesulfonic acid and cyclopentanone and then reacted. The reaction is carried out at room temperature for 12 hours, and then the temperature is raised to 45°C for 3 hours; the reaction After completion, the temperature was lowered to room temperature, and then n-hexane was added dropwise to the reaction system, and compound 2 was obtained by filtering after the solid was precipitated; the molar ratio of compound 1 and p-toluenesulfonic acid added in this step was 1:0.1-0.3;
[0031] b) Compound 2 undergoes a BoC protection reaction to generate compound 3,
[0032]
[0033] This reaction is carried out in the solvent methylene chloride, compou...
Embodiment 1
[0050] The synthesis of embodiment 1 compound 2
[0051] Add 59g of compound 1 (0.205mol) and 3.5g of p-toluenesulfonic acid into the reaction flask, then add 300ml of cyclopentanone, and stir at room temperature for 12 hours. The reaction was then warmed to 45°C and the reaction was stirred for 3 hours. After TLC detects that the reaction is complete, stop the reaction. 600ml of n-hexane was added dropwise to the system under stirring at room temperature, and a solid precipitated out of the system. After stirring at room temperature for 2 hours, filter, wash the filter cake with 60ml of n-hexane, and vacuum-dry the product at 40°C for 5 hours to obtain 65 grams of compound 2 with a yield of 90 %.
Embodiment 2
[0052] The synthesis of embodiment 2 compound 3
[0053]Add 16.9g of compound 2, 0.5g of DMAP, 35g of triethylamine and 85ml of dichloromethane into the reaction flask, then add 30g of (Boc)20, heat up to 30°C for reaction, and detect the reaction by TLC. Wash, dry over anhydrous sodium sulfate, concentrate the organic phase, and recrystallize with 50ml of ethyl acetate to obtain 22.4g of intermediate 3, yield: 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com