Scylla paramamosain C-type lectin as well as preparation method and application thereof
A technology of Scylla lectin and lectin, which is applied in the field of Scylla lectin C-type lectin and its preparation, to achieve the effects of reducing the harm of antibiotics, small molecular weight, and poor immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Synthesize the gene (SEQ ID No. 2):
[0028] ACGGACGTCAACTCATCAAATACCGAGTGCCACAGCCCTTTCACGGAGGTTGCAGGTCGCTGCTTGCACATTGAAGTCGCCACCACTGGCTCGTGGCACAATATGCGAAAGCTCTGTCAGGACCTTGGGGGTGACCTGGTCAATCTTTCTGATCTGCAATTCTACGGTGACCTCATTTTGTACATTAAAAGTTTACATTTGCCATACGTTCATTTGTGGATCGGTGCCACGGACGAGGCGACGGAGGGCATCTGGATGTGGACAGATGGGACACCCGTCAGGATGGGCACTCCTTACTGGGCCAACTATAAGGACAACGTTCAAATGCCTGCTGGAGGAGAGAATCAAAACTGTGCTATGCTTGATATAAACATGCATTATTATTTCAATGATTATGGCTGTTCGTCACCAGATATAAGTCCGATTTGTGAG
[0029] 2) After adding an enzyme-cut position Ncol / XHOL and HIS tag, it is synthesized to a gene fragment: the whole gene synthesis of the present embodiment is enrichment. Finance Biotechnology (Shanghai) Co., Ltd. (hereinafter referred to as the business);
[0030] The gene fragment obtained by NCOL / XHOL dicase cut carrier PET32A and the above steps. Recovery purification, the bisase-cut gene fragment is connected to the carrier PET32a, to which the product is connected and converted into the E...
Embodiment 2
[0035] This embodiment provides a method of preparing a recombinant protein RSPCTL-2:
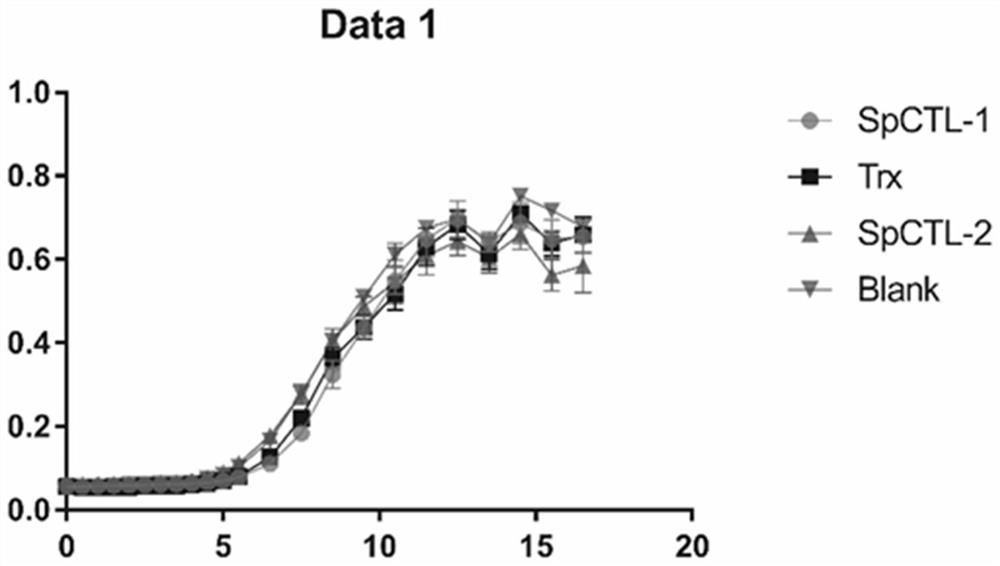
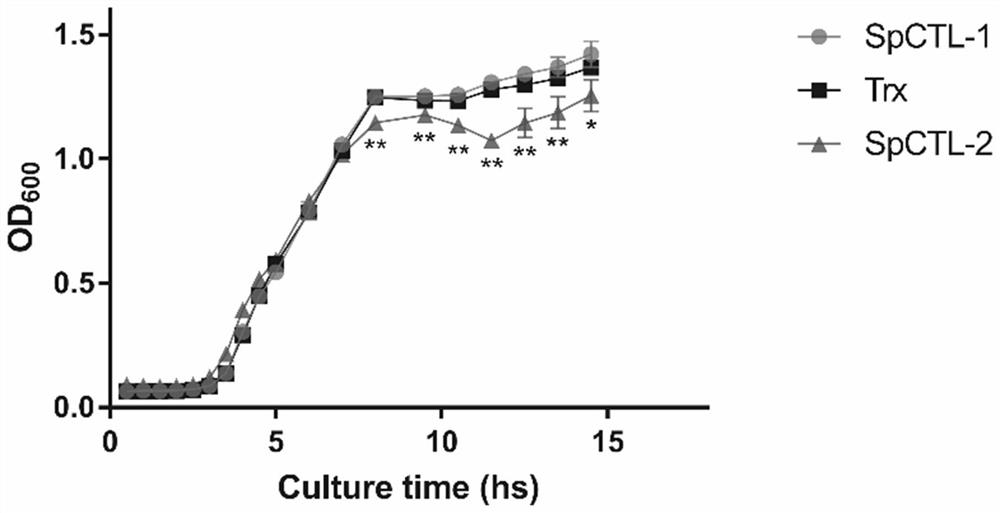
[0036] The recombinant expression vector described in Example 1 was converted to E. coli BL21 (DE3) in the heat excitation, and E. coli strain containing recombinant plasmid was selected, and an E. coli single colony containing recombinant plasmid after antibiotic screening will be selected. It was placed in the LB liquid medium, and 12 h at 37 ° C, 220 rpm was purified; and the bacteria liquid was cultured in the LB medium, 37 ° C, 220 rpm culture was cultured, and 0.01 mM IPTG was induced, and the induced conditions were 30 ° C. , 4H, 220 rpm, a recombinant protein that was prepared by supernatant expression.
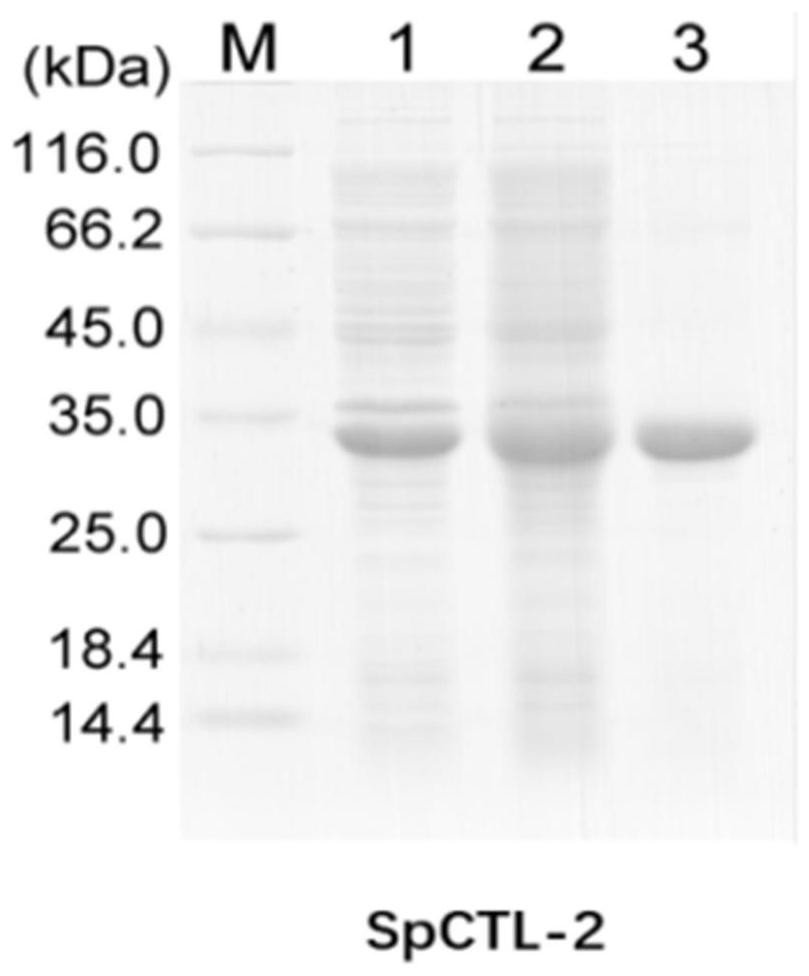
[0037] After collecting induction, the SDS-PAGE was detected with a single strip of about 33 kD.
[0038] Purpose protein was purified by nickel column, and the SDS-PAGE was detected with a single strip of about 33 kD. figure 1 Indicated.
[0039] Among them, the general primer of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com