Preparation method and application of water-soluble cationic polyporphyrin
A technology of water-soluble cations and polyporphyrin, which is applied to medical preparations containing active ingredients, pharmaceutical formulas, photodynamic therapy, etc., can solve the problems of low dark toxicity, weakened PDT efficacy, and short cycle time, so as to improve biological compatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the synthesis of symmetrical type porphyrin monomer (porphyrin monomer 2)
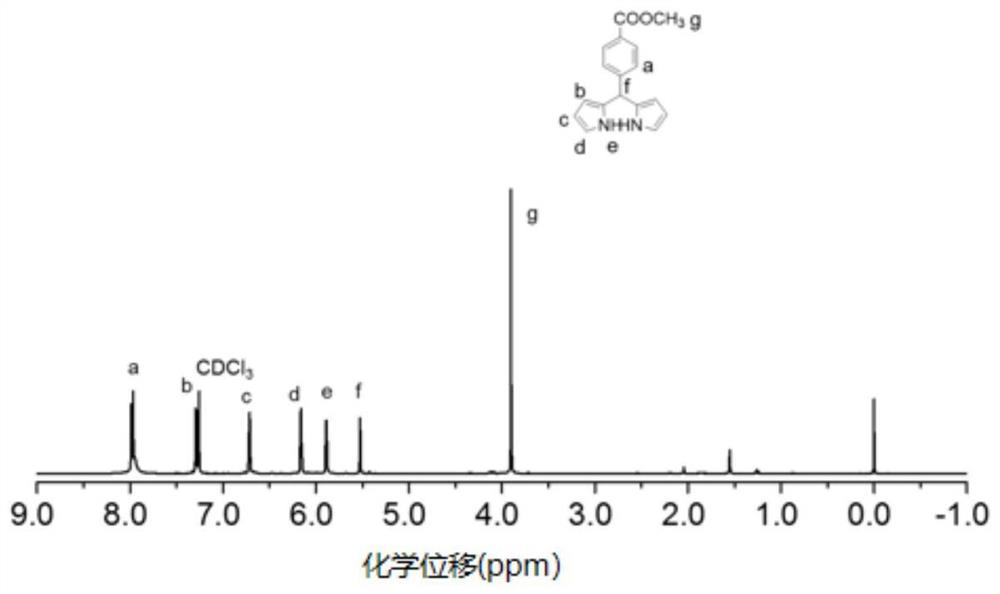
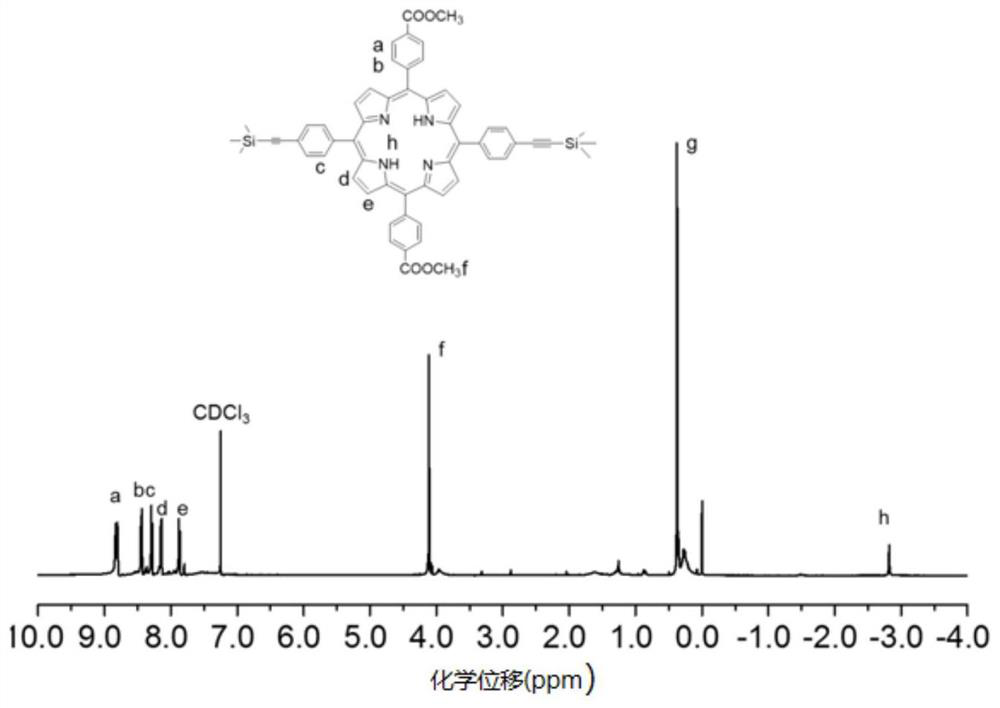
[0034] Weigh methyl p-formylbenzoate (2g, 12.2mmol) and put it in a reaction flask, add pyrrole (4mL, 57.7mmol) dropwise with a needle, stir and dissolve, then add 100μL trifluoroacetic acid dropwise under nitrogen , Continue to stir the reaction at 50°C for 3h, and the reaction is terminated. Separation and purification by column chromatography to obtain the bipyrrole intermediate, the NMR is as follows figure 1 shown. The bipyrrole intermediate was treated and drained, and the bipyrrole intermediate (280.3mg, 1mmol) was weighed and placed in a reaction flask, and then 4-(trimethylsilyl)ethylbenzaldehyde (200mg, 1mmol) was weighed, added In the reaction flask, add 15 mL of dichloromethane to dissolve. After stirring and dissolving, 70 μL of trifluoroacetic acid was added with a needle under nitrogen gas, and the reaction system changed from yellow to deep purple after adding tr...
Embodiment 2
[0035] Embodiment 2: the synthesis of water-soluble cationic polyporphyrin
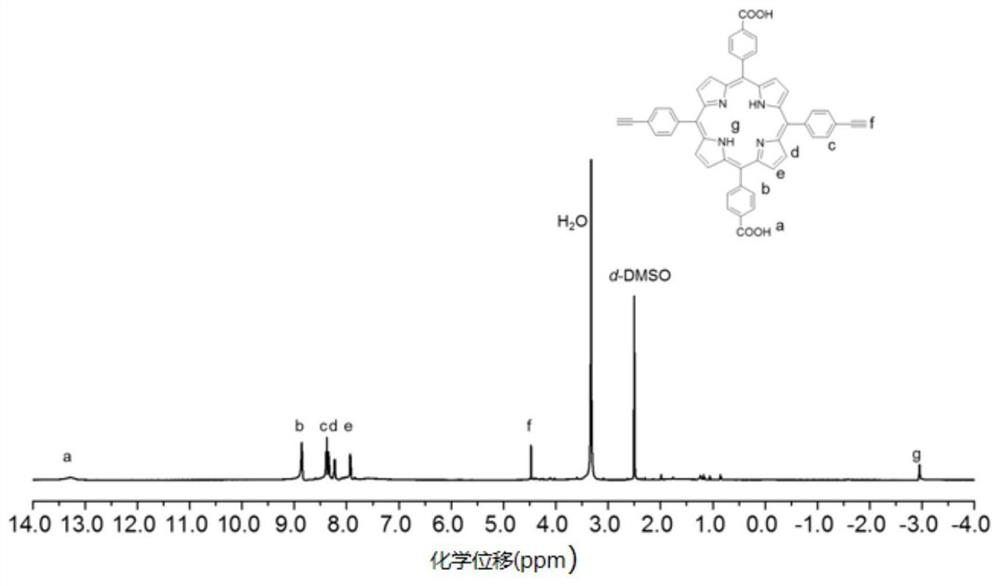
[0036] In the glove box, dissolve porphyrin monomer 2 (150mg, 0.2mmol) and diamine-based monomer (45mg, 0.2mmol) with thioketal bond in DMF, add 1-ethyl-(3- Dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) (184mg, 0.96mmol) and 4-dimethylaminopyridine (DMAP) (96mg, 0.16mmol), reacted at 75°C for 48h, After the reaction was completed, the solvent was removed by rotary evaporation, and the linear polyporphyrin pPS was obtained by sedimentation and centrifugation purification; pPS (100 mg, 0.2 mmol alkynyl) was weighed and dissolved in DMF, and chlorine (pentamethylcyclopentadienyl) (cyclopentadienyl) was added. Octadiene)ruthenium(II)(Cp*Ru(COD)Cl)(3.8mg, 0.01mmol) and 0.6mmol cationic azide were reacted at 90°C for 48h. After the reaction was completed, the sedimentation centrifuged and washed with dichloromethane Water-soluble cationic polyporphyrin is obtained, and the NMR figure is as foll...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com