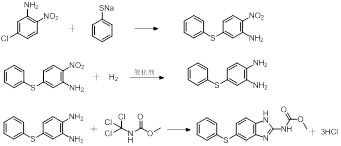
Synthetic method of fenbendazole
A synthesis method and technology for fenbendazole, applied in the field of synthesis of fenbendazole, can solve the problems of difficulty in separation, environmental pollution, and high production costs, and achieve the effects of reducing product quality decline, low cost of three wastes, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of intermediate 5-phenylthio-2-nitroaniline
[0029] Add 172.57g of 5-chloro-2-nitroaniline, 517.71g of n-propanol, 8.00g of sodium hydroxide, and 858.00g of sodium thiophenate aqueous solution into a 1000ml four-necked bottle, stir evenly, and then heat up to 88°C (reflux temperature ), after 5 hours of heat preservation, the temperature was lowered to 0°C while stirring, and filtered after cooling and precipitation, and the filter cake was dried in an oven at 60°C for 5 hours to constant weight to obtain 218.64 g of 5-phenylthio-2-nitroaniline. The yield is 86.14%, and the purity is 97.03%.
[0030] The mass fraction of the sodium thiophenate aqueous solution is 20%.
Embodiment 2
[0031] Embodiment 2 prepares intermediate 5-phenylthio-2-nitroaniline
[0032] Add 172.67g of 5-chloro-2-nitroaniline, 517.71g of n-propanol, 8.00g of sodium hydroxide, and 858.00g of sodium thiophenate aqueous solution into a 1000ml four-necked bottle, replace the system with nitrogen three times and then stir evenly to raise the temperature to 88 ℃, after 5 hours of heat preservation, the temperature was lowered to 0 ℃ while stirring, cooled and precipitated, filtered, and the filter cake was dried in an oven at 60 ℃ for 5 hours to constant weight to obtain 243.50 g of 5-phenylthio-2-nitroaniline. The rate is 98.87%, and the purity is 100%.
[0033] The mass fraction of the sodium thiophenate aqueous solution is 20%.
[0034] Known from embodiment 1, 2, when reacting, system is not carried out nitrogen protection, can cause the yield of 5-phenylthio-2-nitroaniline to obviously reduce, and reason is that sodium thiophenate is easy to deteriorate in air and water, 5-chloro-2...
Embodiment 3
[0035] Embodiment 3 prepares intermediate 5-phenylthio-2-nitroaniline
[0036] Add 172.67g of 5-chloro-2-nitroaniline, 517.71g of n-propanol, 8.00g of sodium hydroxide, and 792.00g of sodium thiophenate aqueous solution into a 1000ml four-necked bottle, replace the system with nitrogen three times, and stir evenly to raise the temperature to 88 ℃, keep warm for 5h, cool and precipitate, then filter, put the filter cake in an oven at 60°C and dry for 5h to constant weight to obtain 242.81g of 5-phenylthio-2-nitroaniline, with a yield of 98.59% and a purity of 100%.
[0037] The mass fraction of the sodium thiophenate aqueous solution is 20%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com