Cyclopeptide capable of resisting multi-drug-resistant bacteria and application of cyclopeptide
A technology for multi-drug-resistant bacteria and multi-drug resistance, applied in the direction of antibacterial drugs, peptides, pharmaceutical formulations, etc., can solve the problems of identifying antibacterial peptides, such as difficulty, and achieve broad-spectrum killing activity, strong operability, and low cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
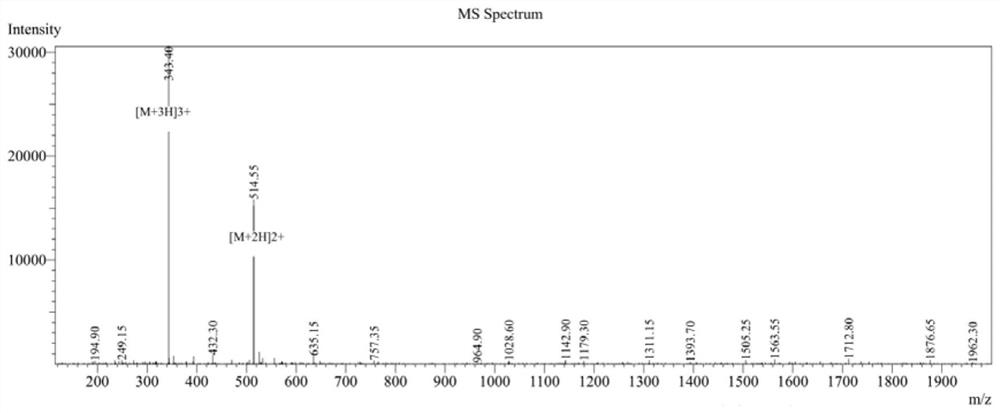
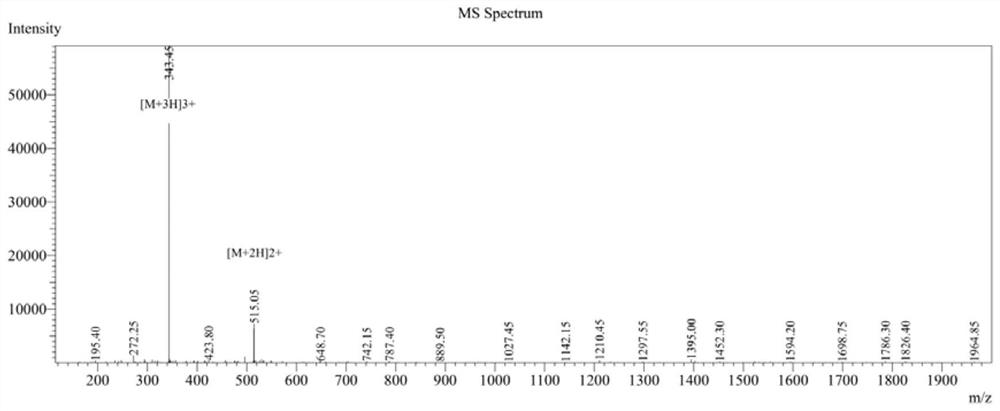
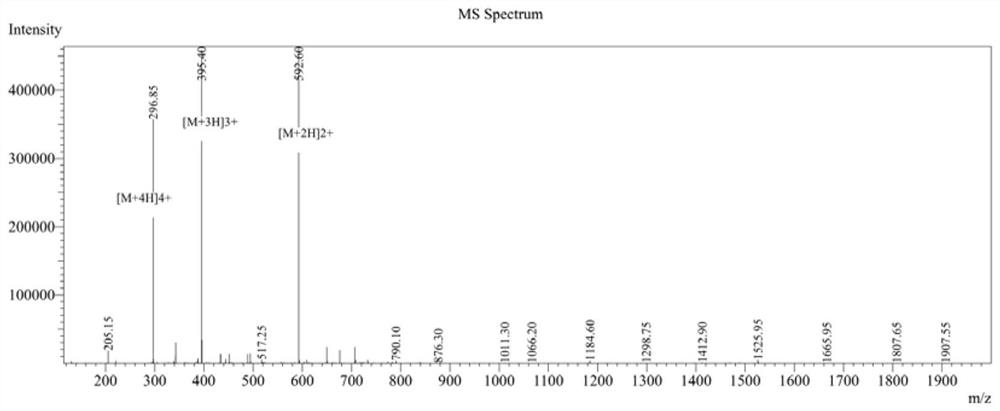
[0049] 1. CPeptide-A: (Arg-Arg-Trp-Trp-Trp-Arg), cyclic peptide synthesis.
[0050] Using Fmoc solid-phase peptide synthesis, with 2-CTC resin as the carrier, the fully protected peptide line peptide is first synthesized, then cyclized in the liquid phase, and finally the crude peptide is obtained by ether precipitation after deprotection with TFA solution.
[0051] Swell 1g of initial resin with 10ml of DCM at room temperature for 30min;
[0052] The first amino acid coupling operation:
[0053] Weigh the protected amino acid Fmoc-Arg(Pbf)-OH with a total resin substitution value of 3eq and add it to the DCM solution, then add DIEA with a total resin substitution value of 9eq for dissolution, and add the dissolved clear solution to the resin for coupling After reacting for 3 hours, the waste liquid was discharged and washed 3 times with DMF.
[0054] The second amino acid coupling operation:
[0055] Remove the Fmoc protecting group: add 5ml 20% PPD / DMF reagent to the reac...
experiment example 1
[0081] Bactericidal activity detection of experimental example 1 cationic antimicrobial cyclic peptide
[0082] Various bacterial strains used in the following examples were purchased from China Institute for the Control of Biological Products.
[0083] Adopt agar punching method to detect the bactericidal activity of cationic antibacterial cyclic peptide, and use the cationic antibacterial peptide LfcinB64-9 RRWQWR synthesized by solid-phase chemical method as contrast, to evaluate CPeptide-A, CPeptide-B, CPeptide-C in the present invention , CPeptide-D, CPeptide-E, CPeptide-F bactericidal activity.
[0084] Measure the bactericidal activity of antimicrobial peptides according to the following steps:
[0085] Strain recovery: inoculate drug-resistant Acinetobacter baumannii, streak in NA nutrient agar medium, and place in a constant temperature incubator for cultivation at a temperature of 37°C for 16-20 hours.
[0086] Strain culture: Pick a single colony and place it in 1...
experiment example 2
[0093] Antibacterial Activity Detection of Experimental Example 2 Cationic Antibacterial Cyclic Peptide
[0094] Various bacterial strains used in the following examples were purchased from China Institute for the Control of Biological Products.
[0095] Determination of the minimum antibacterial ability of cationic antibacterial cyclic peptide, and the cationic antibacterial peptide LfcinB6 synthesized by solid-phase chemical method 4-9 RRWQWR is used as a control to evaluate the antibacterial ability of CPeptide-A, CPeptide-B, CPeptide-C, CPeptide-D, CPeptide-E and CPeptide-F of the present invention.
[0096] The antibacterial activity of the antimicrobial cyclic peptide was determined according to the following steps:
[0097] Collect the bacteria growing in the logarithmic phase, centrifuge at 4°C and 8000 rpm for 2 min, wash with normal saline three times, add fresh broth medium, and make the concentration of the bacterial suspension 2.0×105cfu / mL. Add 50uL of bacterial...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com