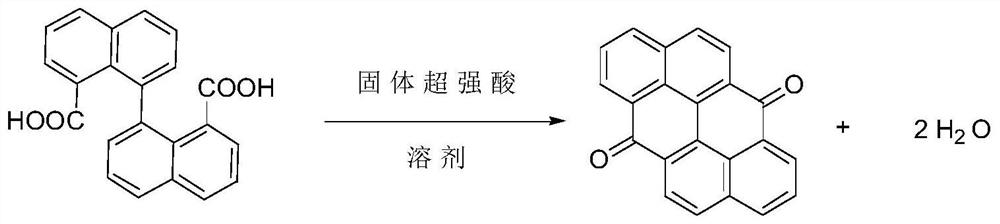
Production method for synthesizing anthracene associated anthrone under catalysis of solid superacid
A technology of solid superacid and anthracenthrone, which is applied in chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve problems such as waste sulfuric acid pollution yield, and achieve the effect of solving pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
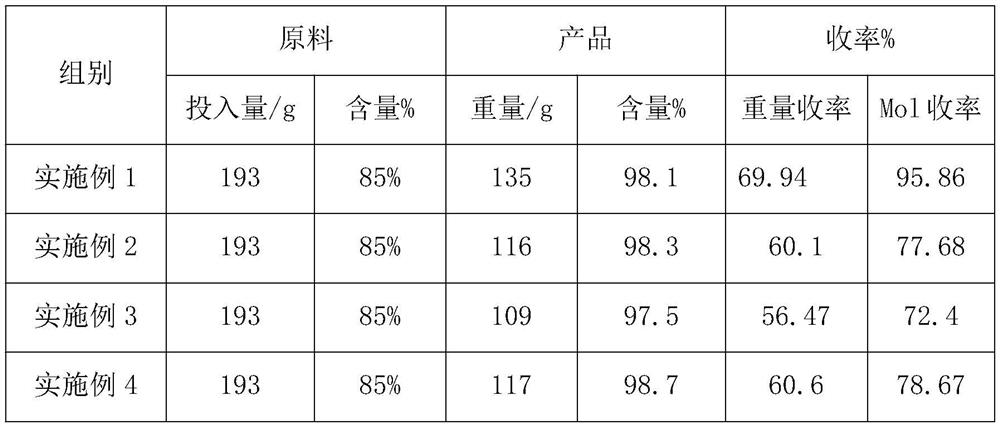
[0021] Ingredients: Add 193 parts of 1,1ˊ-binaphthalene-8,8ˊ-dicarboxylic acid into a 3000L enamel reaction kettle, add 900 parts of glacial acetic acid, start stirring the material and stir evenly, add 350 parts of acetic anhydride and 5 parts of solid super acid ( HND-33).
[0022] Main reaction: heat the reactor to reflux with steam, the material dissolves first, and then a large amount of solids precipitate out. After the reaction is over, distill out glacial acetic acid under reduced pressure. After distilling about 900 parts of glacial acetic acid, stop stirring and continue distilling until there is no liquid flow out. Stop distilling.
[0023] Product purification: slowly add 5% sodium hydroxide aqueous solution to the reaction kettle, slowly start stirring, after stirring for 3 hours, raise the temperature to 95°C, continue stirring until there is no blocky solid in the reaction kettle, then cool down to 55°C , filtered, washed with water until neutral. Dry to obta...
Embodiment 2
[0025] Ingredients: Add 193 parts of 1,1′-binaphthalene-8,8′-dicarboxylic acid into a 3000L enamel reaction kettle, add 1500 parts of o-dichlorobenzene and 5 parts of solid superacid (HND-32).
[0026] Main reaction: heat the reactor to reflux with heat conduction oil, and slowly separate the generated water from the water separator. After the reaction is over, steam distillation is performed, and o-dichlorobenzene is recovered from the bottom of the water separator.
[0027] Product purification: After the distillation of o-dichlorobenzene, slowly add 5% sodium hydroxide aqueous solution to the reaction kettle, slowly start stirring, after stirring for 3 hours, raise the temperature to 95°C, and continue stirring until there is no lump in the reaction kettle Cool to 55° C., filter, and wash with water until neutral. Dry to obtain 116 parts of anthracenthone.
Embodiment 3
[0029] Ingredients: Add 193 parts of 1,1′-binaphthalene-8,8′-dicarboxylic acid into a 3000L enamel reaction kettle, add 1500 parts of chlorobenzene and 5 parts of solid superacid (HND-31).
[0030] Main reaction: Heat the reactor to reflux with steam, and slowly separate the generated water from the water separator. After the reaction is finished, cool down and filter.
[0031] Product purification: put the filter cake containing chlorobenzene back into the 3000L reactor, add 1000 parts of water and 5 parts of tributyl phosphate as defoamer, stir and distill, and separate the chlorobenzene from the water separator. After the chlorobenzene is distilled off, slowly add 5% aqueous sodium hydroxide solution to the reaction kettle to adjust the pH value to 11, stir while hot until there is no lumpy solid in the reaction kettle, cool down to 55°C, filter and wash with water to neutral. Dry to obtain 109 parts of anthracene.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com