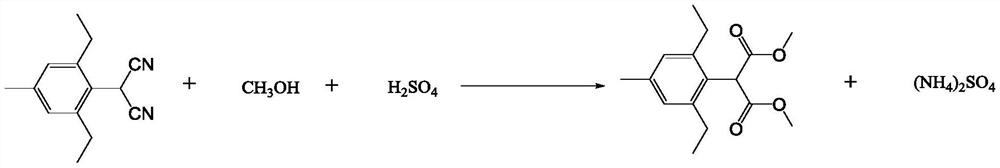Preparation method and application of 2, 6-diethyl-4-methylphenylmalonic acid diester
A technology of methyl phenyl malonate diester and methyl phenyl malononitrile, which is applied in the field of pinoxaden intermediate synthesis, can solve the problems of complex control parameters, plugged microchannel tubes, and large equipment investment, and achieves Good selectivity, good decomposition and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] In some embodiments, the preparation method of the 2,6-diethyl-4-methylphenylmalonate diester comprises: 2,6-diethyl-4-methylphenylmalononitrile, Prepare a mixed solution with inorganic acid, water, organic solvent and alcohol, react at 70-130°C for 6h-11h, then cool, filter and concentrate to obtain the product.
[0046]In some preferred embodiments, the preparation method of the 2,6-diethyl-4 methylphenylmalonic acid diester comprises: 2,6-diethyl-4 methylphenylmalonate Nitrile, inorganic acid, water, organic solvent, alcohol to prepare a mixed solution, react at 80-110°C for 6h-11h, then cool, filter and concentrate to obtain the product.
[0047] In some more preferred embodiments, the preparation method of the 2,6-diethyl-4 methylphenylmalonate diester comprises: 2,6-diethyl-4 methylphenylpropane Dinitrile, inorganic acid, water, organic solvent, and alcohol are prepared to obtain a mixed solution, reacted at 80-95°C for 6h-8h, and then cooled, filtered and concen...
Embodiment 1
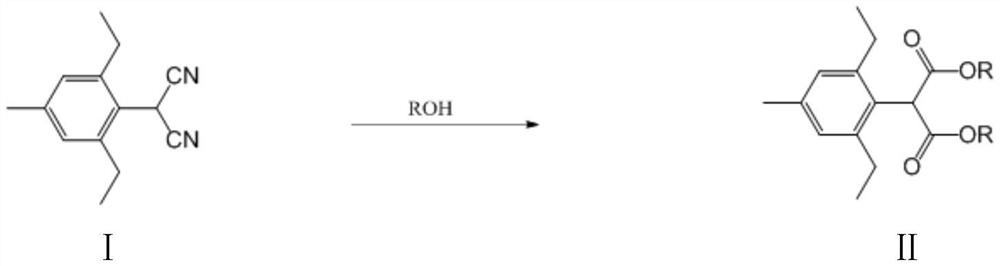
[0051] A kind of preparation method of 2,6-diethyl-4 methylphenylmalonate diester, its general reaction formula is as follows:
[0052]
[0053] In 500mL reactor, add xylene 220g, concentrated sulfuric acid (11g, 0.11mol), water (3.6g, 0.2mol), 2-(2,6-diethyl-4-methylbenzene) malononitrile (22g, 0.1mol), methanol (7.08g, 0.22mol) to start stirring, and stir until the system is clear. Turn on the heating to raise the temperature, keep the internal temperature of the reaction system at 80-95°C, and keep the reaction for 6 hours. After the reaction was completed, it was cooled to room temperature and filtered, and the filtrate was concentrated to obtain 2,6-diethyl-4-methylphenylmalonate with a weight of 26.4 g, a purity of 97.1%, and a yield of 92.1%.
[0054] The product is a white solid with a melting point of 54°C-55°C. 1 HNMR (CDCl 3 ): δ1.18(t, 6H), 2.30(s, 3H), 2.64(q, 4H), 3.73(s, 6H), 5.06(s, 1H), 6.93(s, 2H); 13 CNMR (CDCl 3 ):15.2,21.1,26.6,51.5,52.6,126.4 ,127...
Embodiment 2
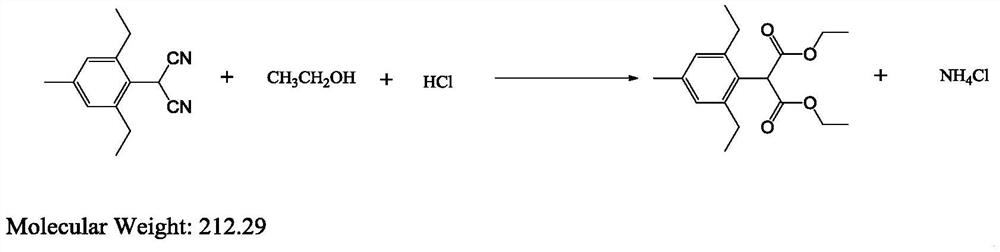
[0056] A kind of preparation method of 2,6-diethyl-4 methylphenylmalonate diester, its general reaction formula is as follows:
[0057]
[0058] Add xylene 220g, water (3.6g, 0.2mol), 2-(2,6-diethyl-4-methylbenzene) malononitrile (22g, 0.1mol), ethanol (11.5 g, 0.25mol) to start stirring, stirring until the system is clear. Dry hydrogen chloride (8 g, 0.22 mol) was passed through. Turn on the heating to raise the temperature, keep the internal temperature of the reaction system at 95-110°C, keep the temperature for 8 hours, cool to room temperature after the reaction is completed, filter the filtrate, and obtain 2,6-diethyl-4-methylphenylmalonic acid di The weight of ethyl ester is 29.07g, the purity is 95.9%, and the yield is 91%.
[0059] The product is a colorless oil, 1 HNMR (CDCl 3 ): δ1.19(t, 6H), 1.27(t, 6H), 2.30(s, 3H), 2.6 5(q, 4H), 4.22(m, 4H), 5.02(s, 1H), 6.92(s , 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com