Morinda officinalis iridoid compounds with anti-inflammatory activity, preparation method and application thereof
A technology for iridoids and iridoid glycosides is applied in the field of Morinda officinalis iridoid compounds and their preparation, which can solve the problems of non-utilization, pollution, waste of resources and the like, and reduce inflammatory response. , the effect of reducing environmental pollution problems and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Extraction and Separation of Partial Iridoid Glycoside Compounds in Morinda officinalis
[0065] The stems and leaves of the aerial part of Morinda officinalis were collected from July to August 2019 at the GAP planting base of Morinda officinalis in Deqing County, Zhaoqing City, Guangdong Province. It was identified as Morinda officinalis by researcher Ding Ping of Guangzhou University of Traditional Chinese Medicine. The stems and leaves of the aboveground part of Morinda officinalis How, the certificate specimen (20190731001) is deposited in the Department of Traditional Chinese Medicine Resources, Guangzhou University of Traditional Chinese Medicine.
[0066] 1. Experimental method
[0067] 1. Extraction and separation of compounds
[0068] After drying the aboveground part of Morinda officinalis, take 10.0kg and cut it off, extract it three times with 95% ethanol (10L×3), seven days each time, and reflux the residue with 95% ethanol again to fully extrac...
Embodiment 2
[0074] The structural identification of embodiment 2 iridoids
[0075] 1. Structural identification of compound Ⅰ
[0076] Compound I is a colorless oil, Ion peak m / z[M+Na] given by HRESIMS mass spectrometry data + 279.1451 (calcd for C 13 h 20 o 5 Na, 279.1203) binding 13 C NMR data can determine that its molecular formula is C 13 h 20 o 5 , with an unsaturation of 4. 1 H NMR shows 1 aldehyde group δ in the low field region H 9.73(1H,s,H-8); 1 pair of double bond δ H 5.99(1H, dd, J=2.4, 5.6Hz, H-3), δ H 5.80 (1H, dd, J=2.4, 5.6Hz, H-2); 1 butoxyl δ H 4.13(1H,m,H-10a), 4.04(1H,m,H-10b), 1.62(2H,m,H-11), 1.37(2H,m,H-12) and 0.92(3H,t, J=7.2Hz, H-13). From 13 C NMR spectrum, DEPT spectrum and HSQC spectrum show 13 carbon signals, including 1 aldehyde group δ C 200.4 (C-8); 1 ester carbonyl δ C 173.5(C-9); 1 pair of double bond δ C 133.4(C-2), 137.2(C-3); 1 continuous oxygen quaternary carbon δ C 84.9 (C-1); 2 oxymethylene signals δ C 68.2 (C-6), 65.1 ...
Embodiment 3
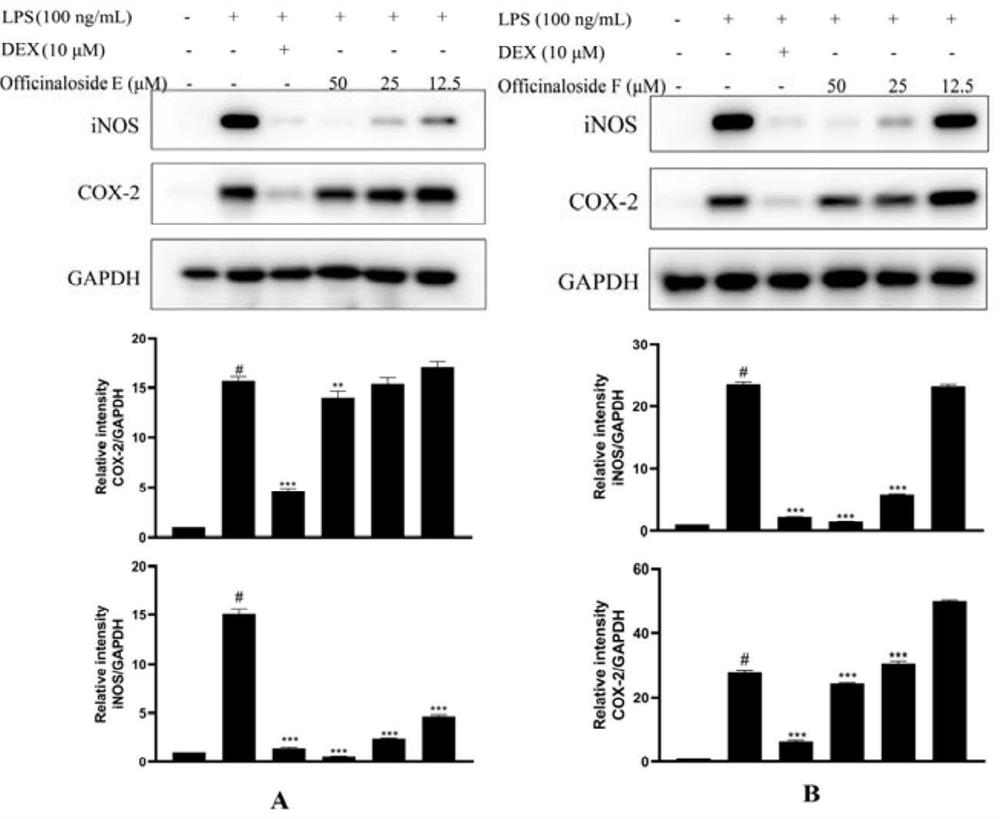
[0094]Example 3 Anti-inflammatory activity experiment of iridoids
[0095] In this example, the in vitro anti-inflammatory activity research experiment of compounds I (officinaloside E) and II (officinaloside F) on LPS-induced RAW 264.7 cells was carried out.
[0096] 1. Experimental materials and instruments
[0097] Clean bench (Suzhou Antai Air Technology Co., Ltd.); constant temperature CO 2 Cell incubator (Germany Heraeus); electric constant temperature water bath (Shanghai Boxun Industrial Co., Ltd.); inverted microscope (Germany LEICA); PAGE vertical electrophoresis instrument (BIO-RAD, USA); fluorescence imaging system, Trizol lysate (Tiangen Biochemical Technology); ultra-micro spectrophotometer (IMPLEN, Germany); 4°C refrigerated centrifuge (Hettich, Germany); metal bath Heater (Hangzhou Aosheng Instruments); PBS phosphate buffer, nitric oxide kit, BCA protein quantification kit, SDS cell lysate, primary antibody diluent, PMSF protease inhibitor, 5 × loading buffer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com