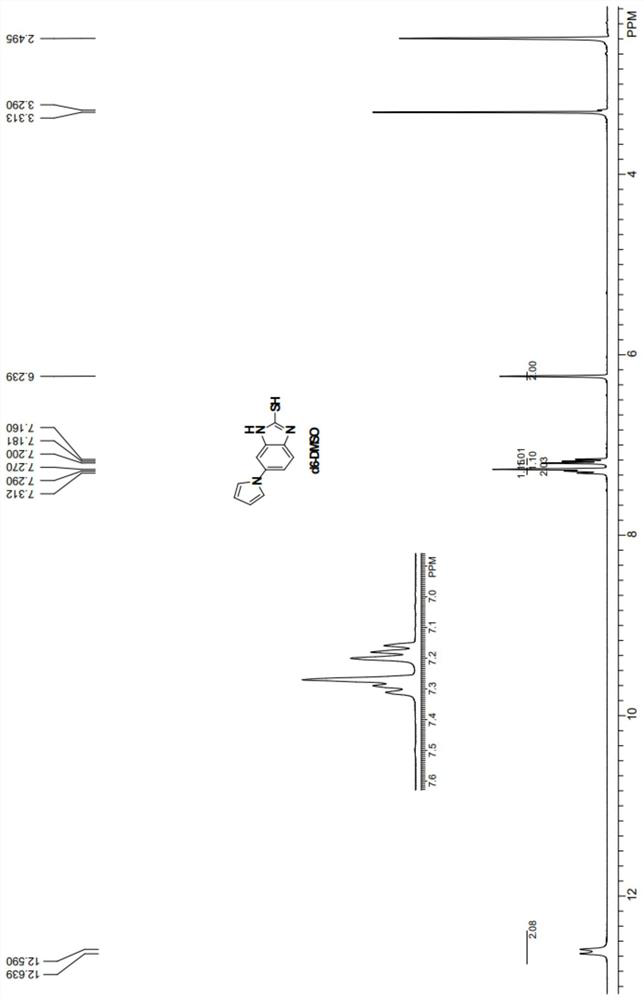
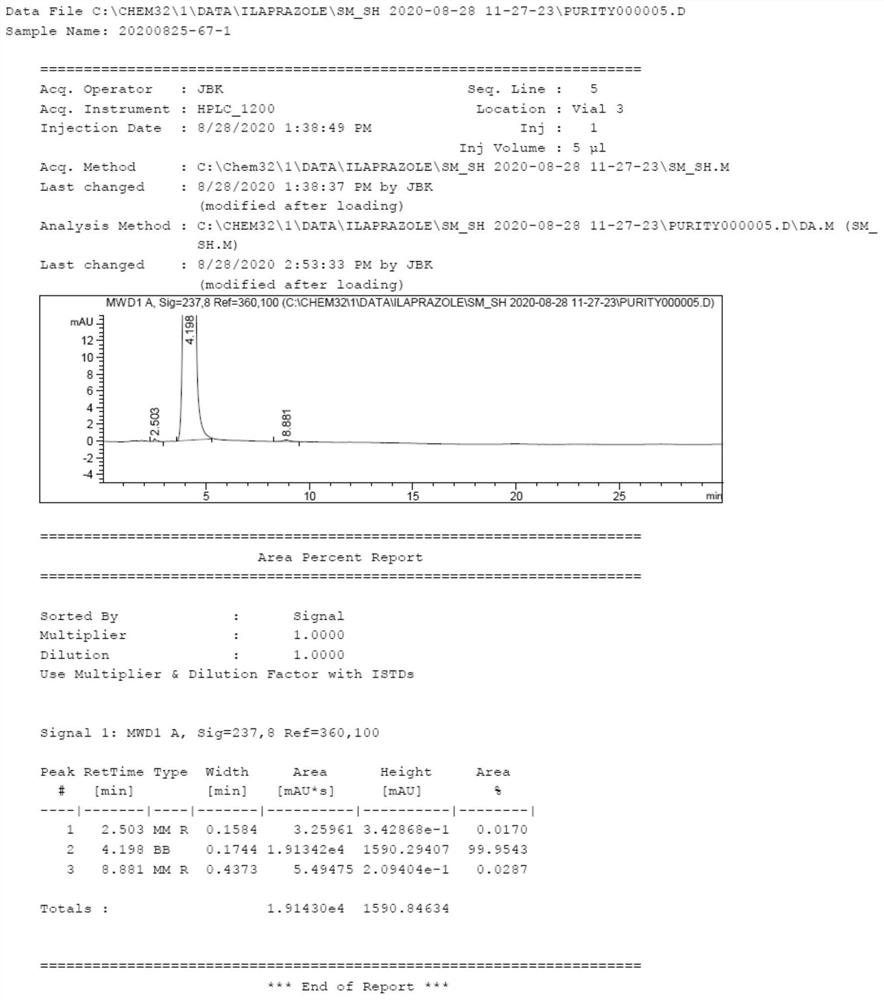
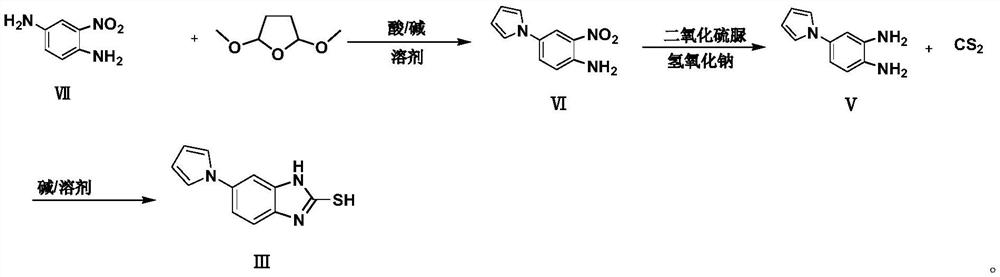
Preparation method of ilaprazole key intermediate 5-(1H-pyrrole-1-yl)-2-mercaptobenzimidazole
A technology of mercaptobenzimidazole and ilaprazole, applied in directions such as organic chemistry, can solve problems such as troublesome processing of reducing agents, expensive metal catalysts, unfavorable industrial production, etc., and achieves low price, stable yield, and easy control of the reaction process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The reaction formula of embodiment 1 is as follows:
[0060]
[0061] 2-Nitro-1,4-phenylenediamine, intermediate VII (100g, 1.0eq) was added to tetrahydrofuran THF (500ml), and sodium acetate trihydrate (88.9g, 1.0eq), acetic acid (196g, 5.0eq), add water (500ml), finally add 2,5-dimethoxytetrahydrofuran (129.5g, 1.5eq), replace with nitrogen, heat up to reflux, react at 60°C for 18 hours, cool to 40°C, add water ( 1.6L, 16.0v / w), the aqueous phase was extracted 4 times with ethyl acetate (500ml), the organic phase was washed once with water (1.0L), methanol (500ml) was added to the crude product and refluxed for 1.5 hours, and water (160ml, 1.6v / w) and reflux for 1.0 hour, then lower the temperature (0-10°C) and stir for 1 hour, and dry the solid in vacuum at 50°C for 18 hours to obtain 120g of red solid 2-nitro-4-(1H-pyrrol-1-yl) Aniline is intermediate Ⅵ with a yield of 90.2%;
[0062] Add ethanol (1.0L), 2-nitro-4-(1H-pyrrol-1-yl) aniline, intermediate VI (100g...
Embodiment 2
[0066] The reaction formula of embodiment 2 is as follows:
[0067]
[0068] 2-Nitro-1,4-phenylenediamine, intermediate VII (100g, 1.0eq) was added to tetrahydrofuran THF (500ml), and sodium acetate trihydrate (88.9g, 1.0eq), acetic acid (196g, 5.0eq), add water (500ml), finally add 2,5-dimethoxytetrahydrofuran (172.6g, 2.0eq), replace with nitrogen, heat up to reflux, react at 62.5°C for 18 hours, cool down to 40°C, add water (1.6L, 16.0v / w), extract the aqueous phase with ethyl acetate (500ml) for 4 times, wash the organic phase with water (1.0L) once, add methanol (500ml) to the crude product and reflux for 1.5 hours, then add water (160ml , 1.6v / w) and reflux for 1.0 hour, then lower the temperature (0-10°C) and stir for 1 hour, and dry the solid in vacuum at 50°C for 18 hours to obtain 121g of red solid 2-nitro-4-(1H-pyrrole-1- Base) aniline is intermediate VI, yield 90.7%;
[0069] Add ethanol (1.0L), 2-nitro-4-(1H-pyrrol-1-yl) aniline, intermediate VI (100g, 1.0eq)...
Embodiment 3
[0073] The reaction formula of embodiment 3 is as follows:
[0074]
[0075] 2-Nitro-1,4-phenylenediamine, intermediate VII (100g, 1.0eq) was added to tetrahydrofuran THF (500ml), and sodium acetate trihydrate (88.9g, 1.0eq), acetic acid (117.6g ,3.0eq), add water (500ml), finally add 2,5-dimethoxytetrahydrofuran (129.5g, 1.5eq), replace with nitrogen, heat up to reflux, react at 65°C for 18 hours, cool to 40°C, add Water (1.6L, 16.0v / w), extracted the aqueous phase with ethyl acetate (500ml) 4 times, the organic phase was washed once with water (1.0L), added methanol (500ml) to the crude product and refluxed for 1.5 hours, then added water ( 160ml, 1.6v / w) and reflux for 1.0 hour, then lower the temperature (0-10°C) and stir for 1 hour, and dry the solid under vacuum at 50°C for 18 hours to obtain 110g of red solid 2-nitro-4-(1H-pyrrole-1- Base) aniline is intermediate VI, and the yield is 83%;
[0076] Add ethanol (1.0L), 2-nitro-4-(1H-pyrrol-1-yl) aniline (100g, 1.0eq)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com