Purine/pyrimidine nucleoside phosphorylase tandem expression engineering bacterium and application
A technology for pyrimidine nucleoside phosphorylase and pyrimidine nucleoside phosphorylase gene, which is applied to purine/pyrimidine nucleoside phosphorylase tandem expression engineering bacteria and application fields, and can solve the problem of low activity, unengineered application and low recovery rate And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Construction of Purine / Pyrimidine Nucleoside Phosphorylase Tandem High-expression Engineering Bacteria
[0022] Include the following steps:
[0023] Step S1: Extract the bacterial genome
[0024] Inoculate Escherichia coli in LB liquid medium and activate overnight at 37°C. The genome was extracted from Escherichia coli, and the steps were carried out according to the Ezup Column Bacterial Genome DNA Extraction Kit.
[0025] Step S2: Amplification of the target gene
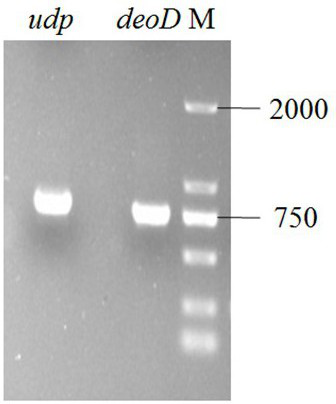
[0026] According to existing in Genbank deoD and udp Gene design primers, design restriction sites according to the map of the plasmid pMD18-T and the target plasmid, and use the extracted genome as a template to amplify the target fragment.
[0027] The 50 μL amplification system is: 0.5 μL of LA Taq enzyme, 5 μL of 10×PCR buffer, 8 μL of dNTP, 1 μL of gene DNA, 1 μL of each primer, ddH 2 O supplemented to 50 μL.
[0028] Amplification conditions were: pre-denaturation at 95 °C for 5 min, and th...
Embodiment 2
[0050] Protein
[0051] Specific steps are as follows:
[0052] Step S1: Induction of engineered bacteria
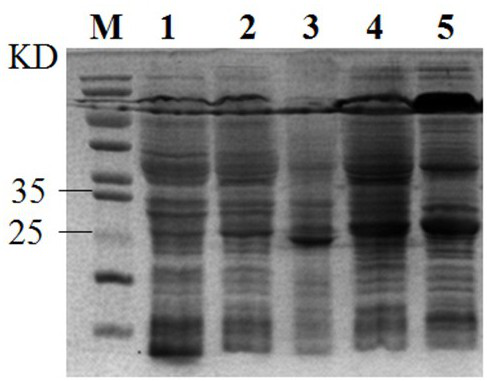
[0053] engineering bacteria E. coli ( deoD ) 、E. coli ( udp ), E. coli ( deoD-udp ) inoculated in LB (Amp + ) liquid, cultured at 37 ºC, inoculated with the original strain and the original strain containing no load at the same time as a control.
[0054] Inoculate LB or LB (Amp + ) liquid, cultured at 37 °C, when the bacterial liquid grows to the pre-logarithmic phase, add IPTG or lactose to a final concentration of 0.01-0.5 mM, and induce at 25-40 °C for 5-10 h.
[0055] Step S2: Preparation of crude enzyme solution
[0056] Centrifuge the induced bacterial solution with a 50 mL centrifuge tube at 6000 rpm for 5 min, discard the supernatant, collect the bacterial cells, and wash 2-3 times with 50 mM Tris-HCl buffer solution of pH 7.5.
[0057] Weigh 0.1 g of wet bacteria into a centrifuge tube, add 1 mL of Tris-HCl buffer to resuspend the bacteria, a...
Embodiment 3
[0061] Enzyme activity detection
[0062] Step S1: PNPase activity detection
[0063] The reaction system is as follows: 2.5 mL of reaction solution (20 mM inosine, 100 mM potassium dihydrogen phosphate, pH 7.0, 10 wt% crude enzyme solution), react at 60 °C for 10 min, add 2 mL of 1 M sodium hydroxide pre-cooled at 4 °C Stop the reaction.
[0064] The reaction results were detected by HPLC. The chromatographic conditions were as follows: Zorbax Eclipse XDB-C18 chromatographic column (4.6 mm×250 mm, 5 μm), and the mobile phase was KH 2 PO 4 Buffer (pH 4.0 50 mM): methanol=92:8, flow rate: 1.0 ml / min; column temperature: 20 ℃; detection wavelength: 259 nm; injection volume: 20 μL. Hypoxanthine standard solutions with concentration gradients of 5 mM, 10 mM, 15 mM, 20 mM, and 25 mM were prepared respectively, the peak areas corresponding to different concentrations were detected, and the linear regression equation between concentration and peak area was established. The enzyme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com