Preparation method of tryptophan derivative medical intermediate
A technology of derivatives and tryptophan, applied in the field of preparation of tryptophan derivative pharmaceutical intermediates, can solve problems such as unreported preparation methods, achieve easy industrial production, high product yield and purity, and simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
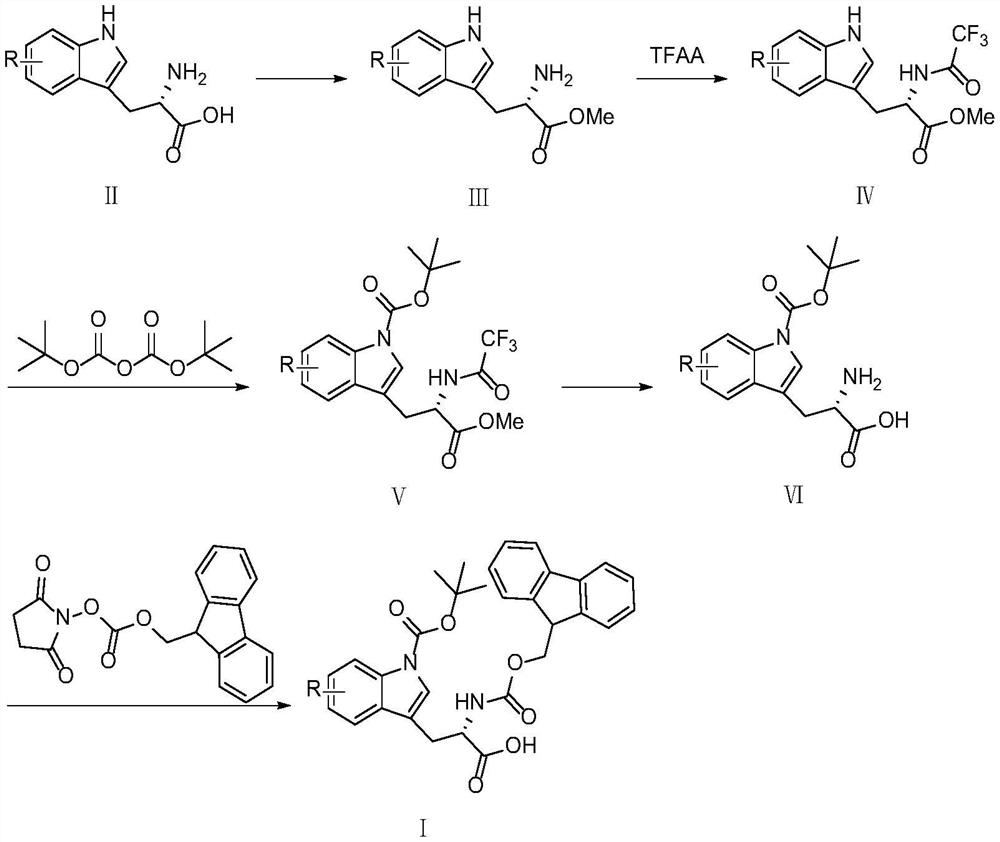
[0044] The synthetic scheme one of preparing tryptophan derivative pharmaceutical intermediate (I) among the present invention is as follows:
[0045]
[0046] (1) Preparation of L-tryptophan methyl ester (Ⅲ-a)
[0047] Add L-tryptophan (4.085g, 20mmol) and 40mL of methanol into a 100mL three-neck flask equipped with magnetic stirring and a thermometer, and slowly add thionyl chloride (4.758g, 40mmol) dropwise under an ice bath at -10°C. Ensure that the temperature of the reaction solution is below room temperature, and after the dropwise addition is completed, the temperature is raised to reflux for esterification reaction, and TLC monitors the end of the reaction. Cool, concentrate to remove methanol, add saturated sodium bicarbonate solution to alkalinize to pH 8-9, add dichloromethane for extraction (3×20ml), take the organic layer and wash with water (2×15ml), dry over anhydrous sodium sulfate, and concentrate Finally, 4.348 g of yellow oily substance L-tryptophan met...
Embodiment 2
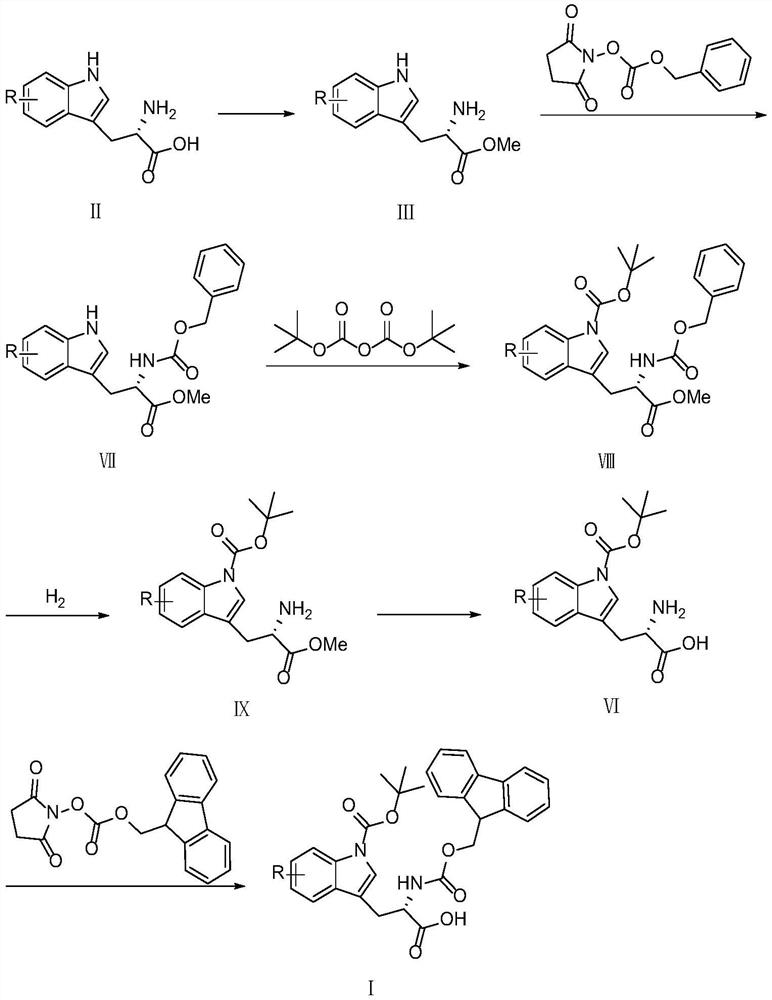
[0087] The synthetic scheme two of preparing tryptophan derivative pharmaceutical intermediate (I) among the present invention is as follows:
[0088]
[0089] (1) Preparation of L-tryptophan methyl ester (Ⅲ-a)
[0090] The preparation process of L-tryptophan methyl ester (III-a) in Example 2 was repeated in Example 1.
[0091] (2) Preparation of N-alpha-benzyloxycarbonyl-L-tryptophan methyl ester (VII-a)
[0092] Add L-tryptophan methyl ester (2.183g, 10mmol) and 20mL of tetrahydrofuran into a 100mL two-necked flask with magnetic stirring and a thermometer, and then dissolve sodium carbonate (1.272g, 12mmol) in water (20ml) to prepare Concentration is about 6% sodium carbonate aqueous solution, then sodium carbonate aqueous solution is added to the reaction solution, after mixing, N-benzylsuccinimide carbonate (2.492g, 10mmol) is added, and the reaction is carried out at room temperature, and TLC monitors the end of the reaction . Separate the layers, wash the organic p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com